(i)
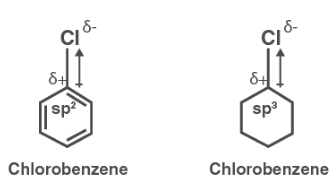
The Cl- atom in chlorobenzene is coupled to an sp2 hybridized carbon atom, whereas it is linked to an sp3 hybridized carbon atom in cyclohexyl chloride. The sp2 hybridized carbon atom is now more electronegative than the sp3hybridized carbon atom because it has more s-character. As a result, in chlorobenzene, the density of electrons in the C-Cl bond near the Cl atom is lower than in cyclohexyl chloride.
Furthermore, the –R effect of chlorobenzene’s benzene ring reduces electron density. As a result, the polarity of the C-Cl bond in chlorobenzene is reduced, and the dipole moment is smaller than in cyclohexyl chloride.
(ii) The miscibility with water is determined when the solute water force of attraction is greater than the solute-solute and water-water forces of attraction. The polar molecules of alkyl halides are held together by dipole-dipole interactions. The forces of attraction between alkyl halides and water molecules are now stronger than the new force of attraction between alkyl halides and water molecules. As a result, alkyl halides (despite their polarity) are insoluble in water.
(iii) Grignard reagents are very reactive. In the presence of moisture, they react to give alkanes.
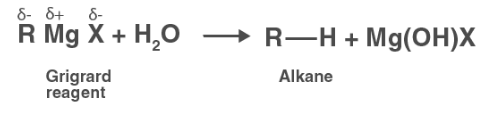
Therefore, Grignard reagents should be prepared under anhydrous conditions.
