(i) protonation of amines gives amide ion.
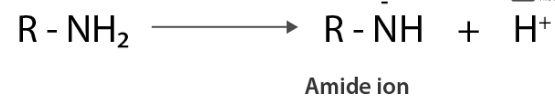
Similarly, alcohol gives away a proton which results in alkoxide ions.
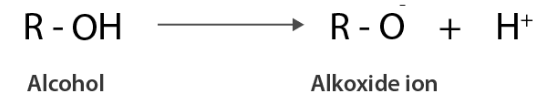
In an amide ion, the negative charge is on the N-atom, whereas in an alkoxide ion, the negative charge is on the O-atom. Due to the fact that O is more electronegative than N, it may easily hold a negative charge. As a result, the alkoxide ion has higher stability than the amide ion. Therefore, alcohols are more acidic than amines of comparable molecular masses.
(ii) In a tertiary amine molecule, there are no hydrogen atoms, whereas primary amines have two hydrogen atoms. Because of the existence of H atoms, primary amines undergo significant intermolecular H – bonding.
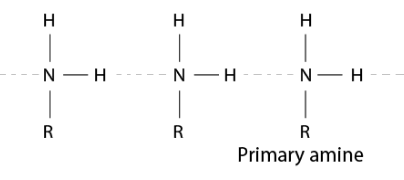
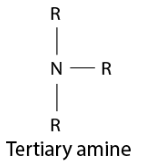
As a result, more energy would be needed to separate the molecules of primary amines. As a result, the boiling temperatures of tertiary amines are lower than those of primary amines.
