(i) When aromatic amine reacts with nitrous acid (which is generated in situ from NaNO2) and a mineral acid like phosphoric acid, it produces nitrous oxide.
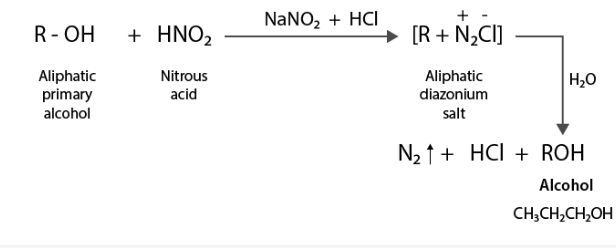
(ii) When aliphatic primary amines react with nitrous acid (produced in situ from NaNO2) and a mineral acid such as HCl, unstable aliphatic diazonium salts develop, which later create alcohol and HCl, as well as N2 gas.
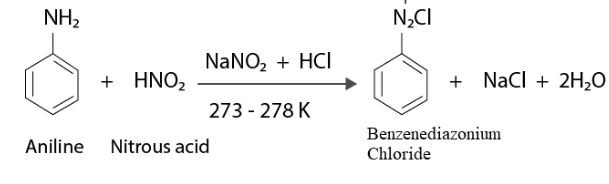
At 273–278 K, (HCl) forms stable aromatic diazonium salts, such as NaCl and H2O.
