The carboxyl group of an amino acid can lose a proton and the amino group can take up a proton in the presence of water or aqueous solution, resulting in a dipolar ion known as a zwitter ion.
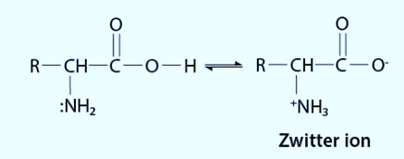
As a result of the presence of zwitterionic form, the amino acid can serve as both an acid and a base.
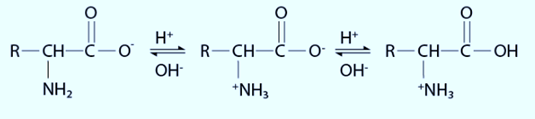
Thus, amino acids are amphoteric in nature.
