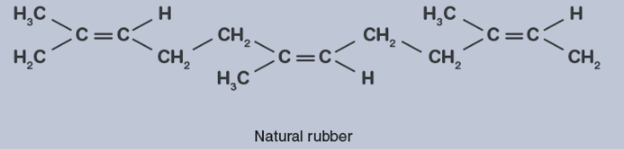
Natural rubber is a cis-polyisoprene with double bonds between the two isoprene units, C2 and C3.
Intermolecular interactions between the multiple isoprene units are relatively weak due to their cis-configuration. Units are randomly organized in natural rubber. As a result, it is flexible.