In [ Ni ( CN)4 ] 2−, Ni has an oxidation state of +2. Thus, it has d 8configuration.
Ni 2+ :
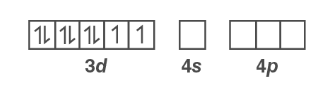
Because CN– is a strong field ligand, electrons in 3d orbitals couple. Ni 2+ undergoes dsp2 hybridization as a result of dsp2 hybridisation.
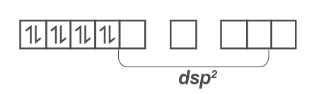
Since all the electrons are paired, it is diamagnetic in nature.
The oxidation state of Cr is +3. It has a d3 arrangement as a result. The electrons in the 3d orbital do not pair because NH3 is not a strong field ligand.
Cr3+:
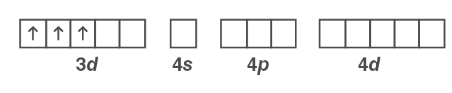
The 3d orbital electrons remain unpaired after d2sp3 hybridization. As a result, [ Ni ( CN)4 ]2 is paramagnetic.
