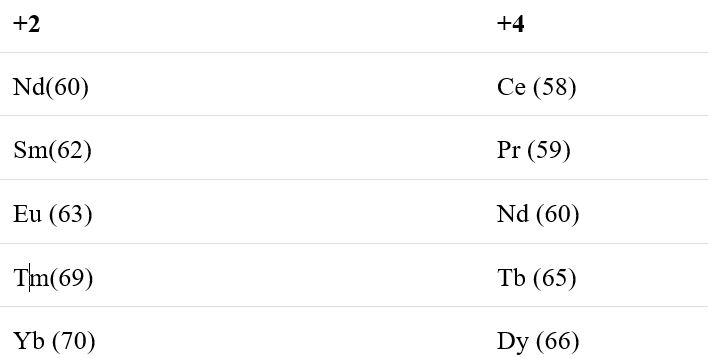
In the parenthesis are the atomic numbers of the elements are given.
Tb after forming Tb4+ attains a stable electronic configuration of [Xe] 4f7.
Yb after forming Yb2+ attains a stable electronic configuration of [Xe] 4f14.
Eu after forming Eu2+ attains a stable electronic configuration of [Xe] 4f7.
Ce after forming Ce4+ attains a stable electronic configuration of [Xe].
