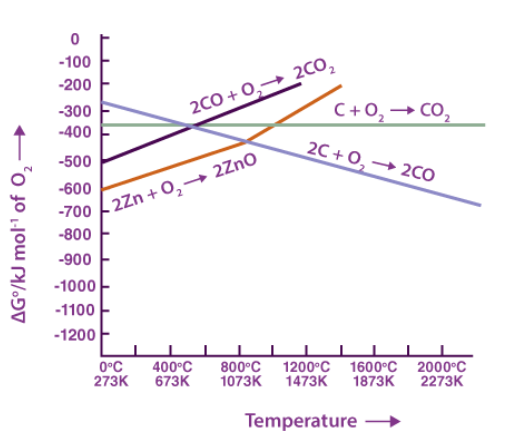
At roughly 1673 K, ZnO is converted to Zn. The Gibbs free energy of formation of CO from C is smaller than the Gibbs free energy of formation of ZnO after 1073 K, and the Gibbs free energy of formation of CO2 from C is smaller than the Gibbs free energy of formation of ZnO beyond 1273 K, as seen in the graph above. As a result, C can convert ZnO to Zn. The Gibbs free energy of CO2 formation from CO, on the other hand, is greater than the Gibbs free energy of ZnO formation. As a result, CO is unable to decrease ZnO, making C a superior ZnO reducing agent.
