Ans:
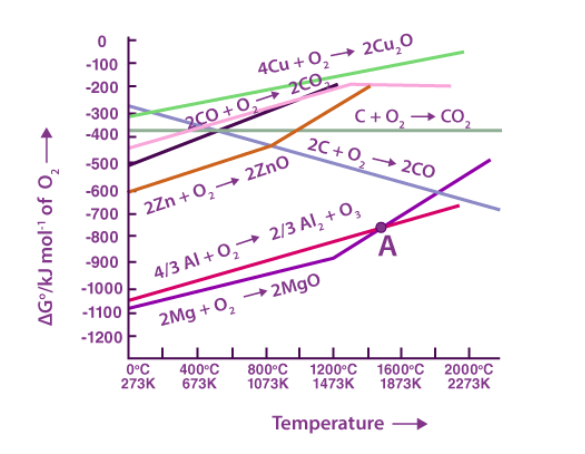
The graph of Gibbs energy ∆Gθ versus Temperature for the formation of solid oxides is shown above. This graph shows that if the ∆fGθ of a metal’s oxide is more negative than the ∆fGθ of another metal’s oxide, the former metal can reduce the latter metal’s oxide (i.e., the oxide with less negative ∆fGθ). For example, because ∆fGθ ( Al, Al2O3) is less negative than ∆fGθ ( Cu, Cu2O), Al can easily decrease Cu2O to Cu but Cu cannot reduce Al2O3. In the same way, Zn cannot reduce MgO, whereas Mg may reduce ZnO to Zn. This is due to the fact that ∆fGθ ( Mg, MgO ) is more negative than ∆fGθ ( Mg, MgO ). ( Zn,ZnO ).
