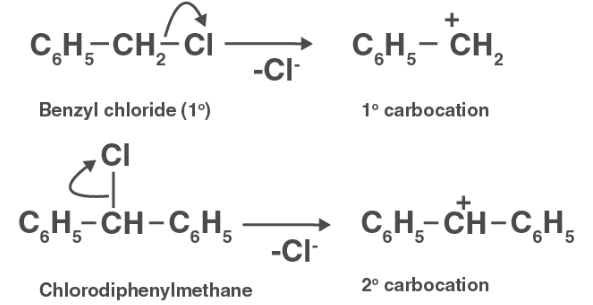
The production of carbocation occurs during aqueous KOH hydrolysis. If the carbocation is stable, aqueous KOH can easily hydrolyze the compound. Now, C6H5 CH2Cl creates 1o– carbohydrate, whereas C6H5CH2ClC6H5 forms 2o–carbohydrate, which is more stable than 1o– carbohydrate. As a result, aqueous KOH hydrolyzes C6H5CHClC6H5 more easily than C6H5 CH2Cl .