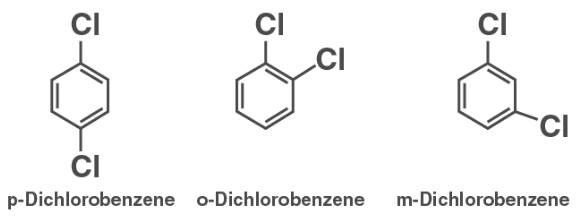
The symmetrical nature of p-Dichlorobenzene is superior to that of the o- and m-isomers. As a result, it matches the crystal lattice better than the o- and m-isomers. As a result, breaking the crystal lattice of p-dichlorobenzene requires greater energy. As a result, p-dichlorobenzene has a lower melting point and is less soluble than the o- and m-isomers.
