(i) 1−bromo−1−methylcyclohexane
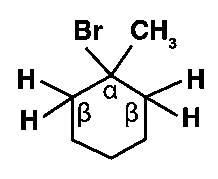
In the given molecule, all β-hydrogens are equivalent. As a result, only one alkene is produced when the given molecule is dehydrogenated.
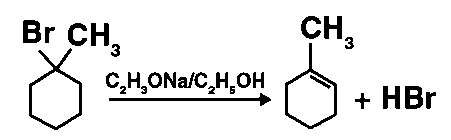
(ii)
Different sets of equivalent -hydrogen atoms are represented by a and b. This chemical yields two alkenes after dehydrohalogenation.
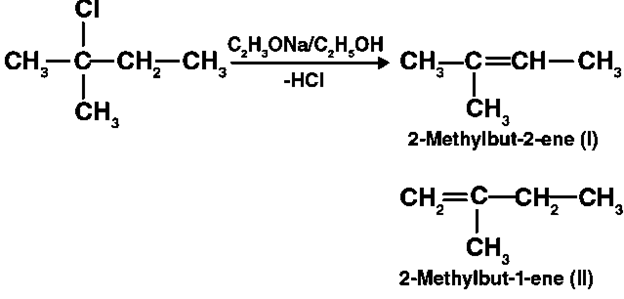
According to Saytzeff’s rule in dehydrohalogenation processes, the alkene with the most alkyl groups connected to a doubly bonded carbon atom produces. As a result, 2-methylbut-2-ene is the main product of this reaction.
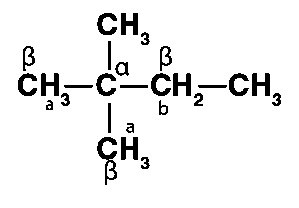
(iii) 2,2,3-Trimethyl-3-bromopentane
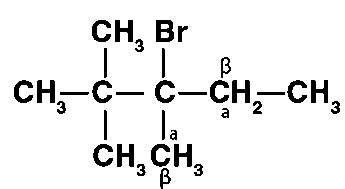
Different sets of equivalent -hydrogen atoms are represented by a and b. This chemical yields two alkenes after dehydrohalogenation.
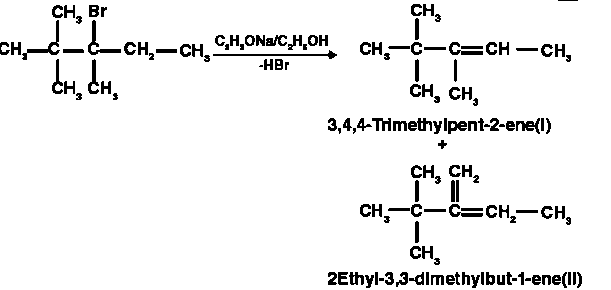
As a result, the main product is 3,4,4-trimethylpent-2-ene.
