The formula C4H9Br is used to make two main alkyl halides. They’re n-butyl bromide and isobutyl bromide, respectively.
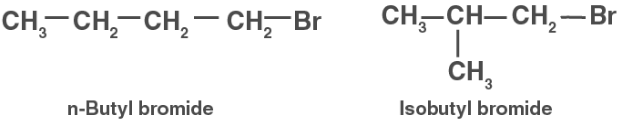
Compound (a) is therefore either nbutyl bromide or isobutyl bromide. Compound (a) now combines with Na metal to generate molecule (b), which has the molecular formula C8H18 and is distinct from the compound formed when nbutyl bromide reacts with Na metal. Isobutyl bromide must therefore be compound (a).
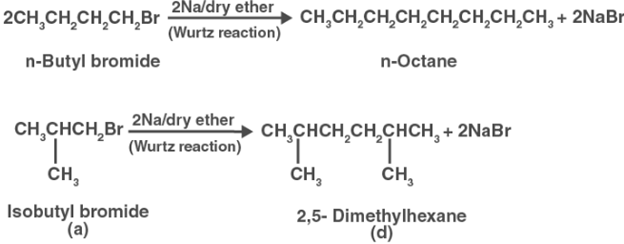
2, 5dimethylhexane is thus compound (d).
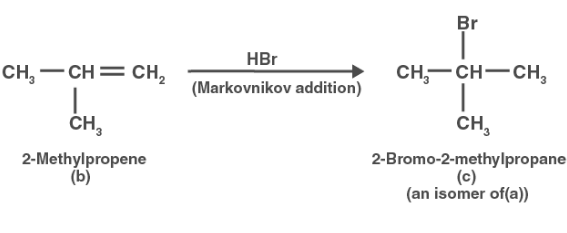
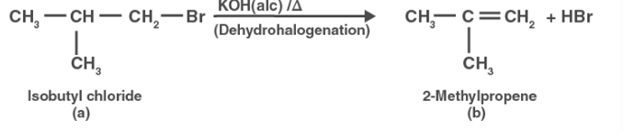
Compound (a) interacts with alcoholic KOH to produce compound (b) (b). Compound (b) is thus 2methylpropene.
Compound (b) also interacts with HBr to form compound (c), which is an isomer of compound (b) (a). Compound (c) is thus 2-Bromo-2-methylpropane.