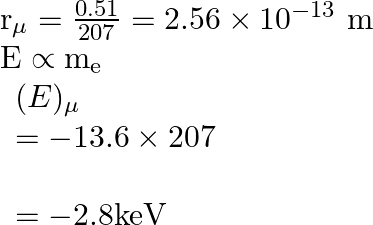The radius of the first permitted Bohr orbit, for the electron, in a hydrogen atom equals
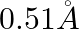
and its ground state energy equals
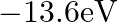
. If the electron in the hydrogen atom is replaced by muon
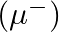
[charge same as electron and mass
![Rendered by QuickLaTeX.com \left.207 \mathrm{~m}_{\mathrm{e}}\right]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-83bf72943cd57fe39ebe177fa39c8114_l3.png)
, the first Bohr radius and ground state energy will be, (1)
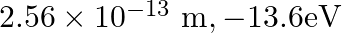
(2)
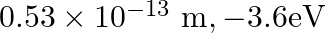
(3)
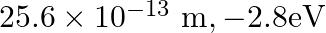
(4)
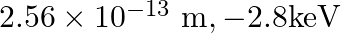
![]()
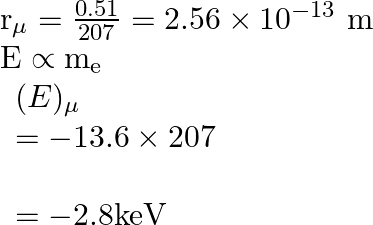
![]()