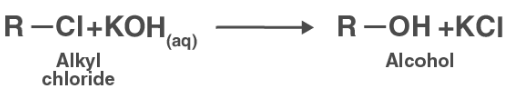
KOH almost entirely ionizes in an aqueous solution, yielding OH ions. Because the OH ion is a powerful nucleophile, it causes the alkyl chloride to undergo a substitution reaction, resulting in the formation of alcohol.
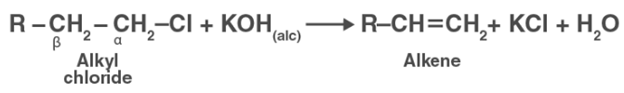
An alcoholic solution of KOH, on the other hand, contains the alkoxide (RO) ion, which is a strong base. By removing a molecule of HCl, it can extract hydrogen from the -carbon of the alkyl chloride and generate an alkene.