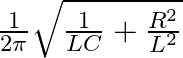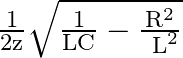The Solution is (3)
![]()
So, ![]()
Two vessels separately contain two ideal gases 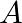 and
and 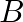 at the same temperature, the pressure of a being twice that of
at the same temperature, the pressure of a being twice that of 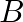 . Under such conditions, the density of
. Under such conditions, the density of 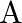 is found to be
is found to be 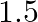 times the density if
times the density if 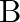 . The ratio of molecular weight of
. The ratio of molecular weight of 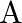 and
and 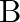 is: (1)
is: (1) 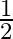 (2)
(2) 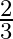 (3)
(3) 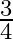 (4) 2
(4) 2