(i) The order in which the halides react to an alkyl group is constant in the SN2 process. This is because as the size of the halide ion rises, it becomes a better leaving group.
R-F << R-Cl << R-Br < R-I
As a result, in SN2 reactions with OH–, CH3I has a faster reactivity than CH3Br.
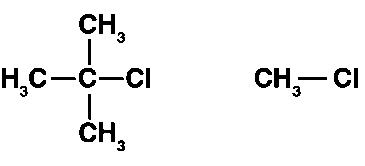
(ii) The nucleophile hits the atom with the leaving group in the SN2 process. The attack of the nucleophile on the carbon atom in (CH3)3CCl is hampered by the presence of bulky substituents on the carbon atoms (carrying the leaving group).
CH3Cl, on the other hand, lacks bulky substituents on the carbon atom that bears the leaving group. As a result, in the SN2 reaction with OH–, CH3Cl has a faster reactivity than (CH3)3Cl.