(i) CH2Cl2 (ii) CHCl3 (iii) CCl4
Solution:
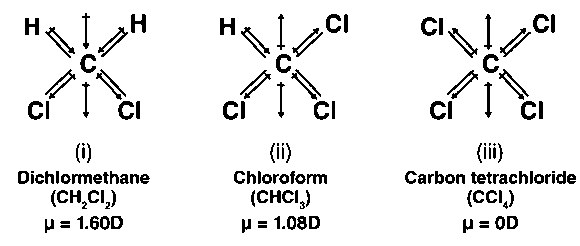
In CHCl3, the resultant of dipole moments of two C – Cl bonds is countered by the resultant of dipole moments of one CH bond and one C – Cl bond, as seen in the above figure. Because the sum of the dipole moments of one C – H bond and one C – Cl bond is less than two, C-CL bonds have a minimal amount of opposition. CHCl3 has a tiny dipole moment of 1.08 D as a result of this. The resultant of the dipole moments of two C – Cl bonds, on the other hand, is enhanced by the resultant of the dipole moments of two C – H bonds in CH2Cl2.
As a result, CH2Cl2 has a 1.60 D higher dipole moment than CHCl3, indicating that CH2Cl2 has the highest dipole moment.
As a result, the following compounds can be grouped in ascending order of dipole moments:
CCl4 < CHCl3 < CH2Cl2
