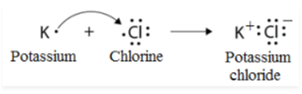
Solution:
The letter K represents element A. (Potassium).
It would be possible to have the electrical configuration of element A (atomic number 19) as 2, 8, 8, 1. Because it has only one valence electron, it must be a metal, according to the definition. As a result, it is potassium.
Element B is represented by the letter Cl (Chlorine).
The electronic configuration of element B (atomic number 17) would be 2, 8, 7, which is the same as the electronic configuration of hydrogen. Because it possesses seven valence electrons, it must be a non-metal, according to the definition. As a result, it is chlorine.
In most cases, an ionic bond is formed between a metal and a nonmetal. Metals have a tendency to shed electrons and produce cations, whereas non-metals have a tendency to take electrons and generate anions.
In the presence of an ionic link, potassium and chlorine will combine to generate potassium chloride (KCl).
The electron dot structure of KCl is depicted in the diagram below: