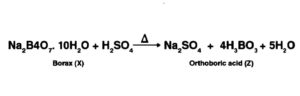
Solution:
The salt given to litmus is antacid. 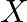 is, subsequently, a salt with a solid base, and a feeble corrosive. When
is, subsequently, a salt with a solid base, and a feeble corrosive. When 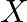 is warmed unnecessarily, it additionally enlarges to frame material
is warmed unnecessarily, it additionally enlarges to frame material 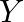 . Subsequently
. Subsequently 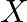 must be borax. When warmed, borax loses water and enlarges to shape sodium metaborate. When warming is proceeded, the framing of a smooth material Y sets. In this way,
must be borax. When warmed, borax loses water and enlarges to shape sodium metaborate. When warming is proceeded, the framing of a smooth material Y sets. In this way, 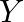 should be a combination of metaborate sodium and boric anhydride.
should be a combination of metaborate sodium and boric anhydride.
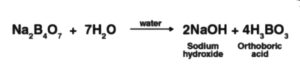
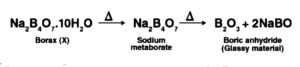
At the point when concentrated corrosive is added to borax, white precious stones of orthoboric corrosive (Z) are framed.