(i) ethyne (ii) ethene (iii) benzene
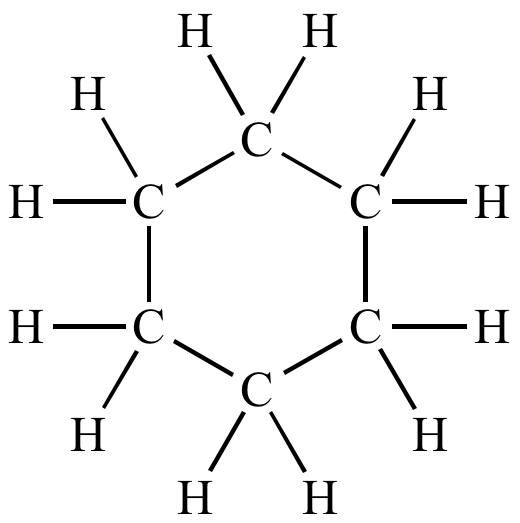
(b) Write the molecular formula and structure of cyclohexane. How many covalent bonds are there in the molecule of cyclohexane?
Solution:
(i)(ii)

(iii)

(i) Ethyne:- Single bonds: Two; Triple bond: One
(ii) Ethene:- Single bonds: Four; Double bond: One
(iii) Benzene: Single bonds C-C: Three; Single bond C-H: Six; Double bonds C=C: Three
(b) C6H12 is cyclohexane. We can see six carbon atoms connected by single bonds and twelve carbon-hydrogen single bonds in the structure of cyclohexane. As a result, there are 18 covalent bonds in the cyclohexane molecule’s structure.