Correct option is
B ![]()
As CN ![]() is a strong ligand so here pairing happens and dsp
is a strong ligand so here pairing happens and dsp ![]() hybridisation (square planar) takes place while
hybridisation (square planar) takes place while ![]() and
and ![]() have sp
have sp ![]() hybridisation (tetrahedral shape).
hybridisation (tetrahedral shape).
Hence, the correct option is B.
Among ![Rendered by QuickLaTeX.com [\mathrm{Ni}(\mathrm{CO}) 4],[\mathrm{Ni}(\mathrm{CN}) 4]^{2-}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-25ca8cc0452b80c10765631cf5f37277_l3.png) and
and ![Rendered by QuickLaTeX.com \left[\mathrm{NiCl}_{4}\right]^{2-}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-ae9f97570b35b9a9ec847eef5963fc9b_l3.png) species, the hybridisation state of
species, the hybridisation state of 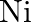 atoms are respectively:
atoms are respectively:
A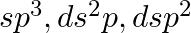
B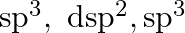
C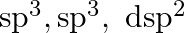
D dsp
dsp 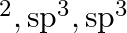
A
B
C
D
Among ![Rendered by QuickLaTeX.com [\mathrm{Ni}(\mathrm{CO}) 4],[\mathrm{Ni}(\mathrm{CN}) 4]^{2-}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-25ca8cc0452b80c10765631cf5f37277_l3.png) and
and ![Rendered by QuickLaTeX.com \left[\mathrm{NiCl}_{4}\right]^{2-}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-ae9f97570b35b9a9ec847eef5963fc9b_l3.png) species, the hybridisation state of
species, the hybridisation state of 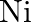 atoms are respectively:
atoms are respectively:
A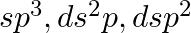
B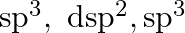
C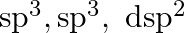
D dsp
dsp 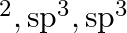
A
B
C
D
