Correct option is B False
Let ![]() be the oxidation number of
be the oxidation number of ![]() in
in ![]() .
.
Since the overall
charge on the complex is 0 , the sum of oxidation states of all elements in
it should be equal to 0 .
Therefore, ![]()
Hence, ![]()
Thus, the oxidation number of ![]() in
in ![]() is 0 .
is 0 .
Hence, the correct option is B
The oxidation number of 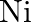 in
in 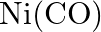 \& is
\& is 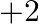 .
.
A True
B False
C Anomalous
D None of these
A True
B False
C Anomalous
D None of these
The oxidation number of 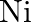 in
in 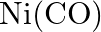 \& is
\& is 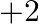 .
.
A True
B False
C Anomalous
D None of these
A True
B False
C Anomalous
D None of these
