(a) What happens when a concentrated solution of sodium chloride (brine) is electrolysed? Write the equation of the reaction involved.
(b) Why is the electrolysis of a concentrated solution of sodium chloride known as chlor-alkali process?
Answer:
(a) When a concentrated sodium chloride solution is electrolyzed, sodium hydroxide, chlorine, and hydrogen are formed.
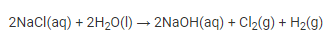
(b) Because of the products formed, chlorine and hydrogen, the electrolysis of a concentrated solution of sodium chloride is known as chlor-alkali.
