(a) Describe how washing soda is produced starting from sodium chloride (common salt). Write equations of all the reactions involved.
(b) What is meant by saying that washing soda has detergent properties?
Answer:
(a) Washing soda is produced from sodium chloride
Step 1: To make sodium hydrogencarbonate, a cold and concentrated solution of sodium chloride called brine is combined with ammonia and carbon dioxide.
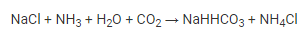
Step 2: The formed sodium hydrogencarbonate is filtered, dried, and heated. It then decomposes further into sodium carbonate.
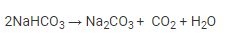
Anhydrous sodium carbonate is called soda ash.
Step 3: The soda ash is dissolved in water and recrystallized to produce washing soda crystals that contain 10 molecules of water during the crystallisation process.
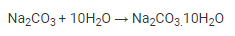
(b) Because washing soda has cleansing and detergent properties, it effectively removes dirt and grease from soiled clothing.
