Solution:
O.N. of S in H2SO5. By traditional strategy, the O.N. of S in H2SO5 is 2 (+1) + x + 5 (- 2) = 0 or x = +8 This is outlandish on the grounds that the most extreme O.N. of S can’t be more than six since it has just six electrons in the valence shell. This error is survived in the event that we ascertain the O.N. of S by synthetic holding technique. The construction of H2SO5 is
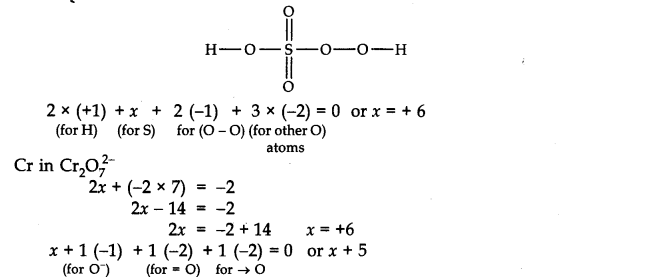
Accordingly, there is no misrepresentation about the O.N. of N in N03–whether one works out by traditional strategy or by synthetic holding technique.
