Solution: The galvanic cell relating to the given redox response can be displayed as: $\mathrm{Zn}\left|Z n_{(a q)}^{2+} \| A g_{(a q)}^{+}\right| \mathrm{Ag}$ (I) Zn anode is contrarily charged on...
Depict the galvanic cell in which the reaction is:
Given the standard electrode potentials, 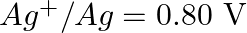
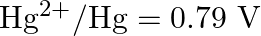
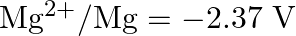
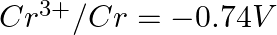 Arrange these metals in their increasing order of reducing power.
Arrange these metals in their increasing order of reducing power.
Solution: The diminishing specialist is more grounded as the terminal potential declines. Subsequently, the expanding request of the lessening force of the given metals is as given underneath: Ag...
Arrange the given metals in the order in which they displace each other from the solution of their salts. Al, Fe, Cu, Zn, Mg
Solution: A metal with more grounded diminishing force uproots one more metal with more vulnerable lessening power from its answer of salt. The request for the expanding diminishing force of the...
Predict the products of electrolysis in each of the following: (i) An aqueous solution of 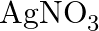 with silver electrodes (ii) An aqueous solution
with silver electrodes (ii) An aqueous solution 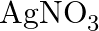 with platinum electrodes (iii) A dilute solution of
with platinum electrodes (iii) A dilute solution of 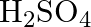 with platinum electrodes (iv) An aqueous solution of
with platinum electrodes (iv) An aqueous solution of 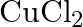 with platinum electrodes.
with platinum electrodes.
Solution: (I) In fluid arrangement, AgNO3 ionizes to give Ag+(aq) and NO3–(aq) particles. \[AgN03\left( aq \right)\text{ }\to \text{ }Ag+\left( aq \right)\text{ }+\text{ }NO3\left( aq...
Using the standard electrode potentials given in Table 8.1, predict if the reaction between the following is feasible: (a) 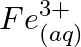 and
and 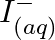 (b)
(b) 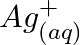 and
and 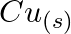 (c)
(c) 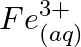 and
and 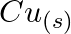 (d)
(d) 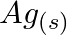 and
and 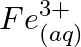 (e)
(e) 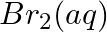 and
and 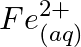
Solution: (a) $F e_{(a q)}^{3+}$ and $I_{(a q)}^{-}$ $2 F e_{(a q)}^{3+}+2 I_{(a q)}^{-} \rightarrow 2 F e_{(a q)}^{2+}+I_{2(s)}$ Oxidation half response: $2 I_{(a q)}^{-} \rightarrow I_{2}(s)+2...
In Ostwald’s process for the manufacture of nitric acid, the first step involves the oxidation of ammonia gas by oxygen gas to give nitric oxide gas and steam. What is the maximum weight of nitric oxide that can be obtained starting only with 10.00 g. of ammonia and 20.00 g of oxygen?
Solution: The reasonable response is as given underneath: $4 \mathrm{NH}_{3(g)}+5 \mathrm{O}_{2}(g) \rightarrow 4 \mathrm{NO}_{(g)}+6 \mathrm{H}_{2} \mathrm{O}_{(g)}$ $4 N H_{3}=4 \times 17...
Refer to the periodic table given in your book and now answer the following questions: (a) Select the possible non – metals that can show disproportionation reaction? (b) Select three metals that show disproportionation reaction?
Solution: One of the responding components consistently has a component that can exist in somewhere around 3 oxidation numbers. (I) The non - metals which can show disproportionation responses are...
Chlorine is used to purify drinking water. Excess of chlorine is harmful. The excess of chlorine is removed by treating with sulphur dioxide. Present a balanced equation for this redox change taking place in water.
Solution: The redox response is as given beneath: Cl2(s)+SO2(aq)+H2O(l)→Cl(aq)-+SO4(aq)2- The oxidation half response: SO2(aq)→SO4(aq)2- Add 2 electrons to adjust the oxidation no. :...
The 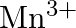 ion is unstable in solution and undergoes disproportionation to give
ion is unstable in solution and undergoes disproportionation to give 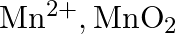 , and
, and 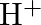 ion. Write a balanced ionic equation for the reaction.
ion. Write a balanced ionic equation for the reaction.
Solution: The response is as given beneath: $\mathrm{Mn}_{(a q)}^{3+} \rightarrow \mathrm{Mn}_{(a q)}^{2+}+\mathrm{MnO}_{2(s)}+\mathrm{H}_{(a q)}^{+}$ The oxidation half response: $\mathrm{Mn}_{(a...
What sorts of informations can you draw from the following reaction? 
Solution: The oxidation no. of $\mathrm{C}$ in $(C N)_{2}, C N^{-}$and $C N O^{-}$are $+3,+2$ and $+4$ separately. Let the oxidation no. of $\mathrm{C}$ be $\mathrm{y}$. $(C N)_{2}$ $2(y-3)=0$ Along...
Balance the following equations in basic medium by ion-electron method and oxidation number methods and identify the oxidising agent and the reducing agent. (a) 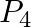 (s)
(s) 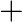
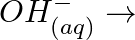
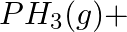
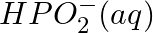 (b)
(b) 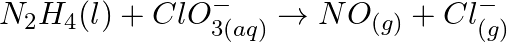 (c)
(c) 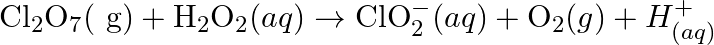
Solution:
Balance the following redox reactions by ion – electron method : (a) 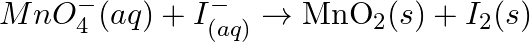 (Basic medium) (b)
(Basic medium) (b) 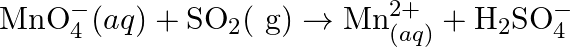 (Acidic medium) (c)
(Acidic medium) (c) 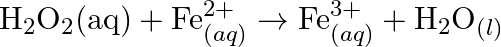 (Acidic medium) (d)
(Acidic medium) (d) 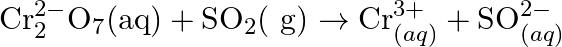 (Acidic medium)
(Acidic medium)
Solution: (a) $M n O_{4}^{-}(a q)+I_{(a q)}^{-} \rightarrow \operatorname{MnO}_{2}(s)+I_{2}(s)$ Stage 1 The two half responses are given beneath: Oxidation half response: $I_{(a q)} \rightarrow...
Consider the reactions: (a) 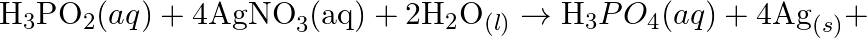
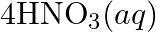 (b)
(b) 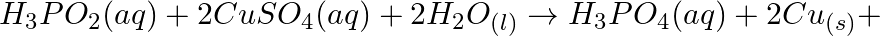
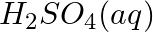 (c)
(c) ![Rendered by QuickLaTeX.com \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CHO}_{(l)}+2\left[\mathrm{Ag}\left(\mathrm{NH}_{3}\right)_{2}\right]_{(a q)}^{+}+3 \mathrm{OH}_{(a q)}^{-} \rightarrow \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{COO}_{(a q)}^{-}+2 \mathrm{Ag}_{(s)}+](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-7aa1b43808014d89aabd5bf76808b357_l3.png)
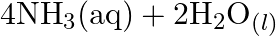 (d)
(d) 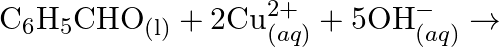 No change is observed What inference do you draw about the behavior of
No change is observed What inference do you draw about the behavior of 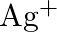 and
and 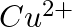 from these reactions?
from these reactions?
Solution: $\mathrm{Ag}^{+}$and $C u^{2+}$ acts as oxidizing specialist in responses (I) and (ii) individually. In response (iii), $\mathrm{Ag}^{+}$oxidizes $\mathrm{C}_{6} \mathrm{H}_{5}...
Why does the following reaction occur?  What conclusion about the compound
What conclusion about the compound 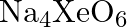 (of which
(of which 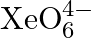 is a part) can be drawn from the reaction?
is a part) can be drawn from the reaction?
Solution: $X e O_{6(a q)}^{4-}+2 F_{(a q)}^{-}+6 H_{(a q)}^{+} \rightarrow X e O_{3(g)}+F_{2(g)}+3 H_{2} O_{(l)}$ The oxidation no. of Xe decreases from $+8$ in $\mathrm{XeO}_{6}^{4-}$ to $+6$ in...
Justify giving reactions that among halogens, fluorine is the best oxidant and among hydrohalic compounds, hydroiodic acid is the best reductant.
Solution: $F_{2}$ can oxidize $C l^{-}$to $C l_{2}, B r^{-}$to $B r_{2}$, and $I^{-}$to $I_{2}$ as: $F_{2(a q)}+2 C l_{(s)}^{-} \rightarrow 2 F_{(a q)}^{-}+C l_{2(g)}$ $F_{2}(a q)+2 B r_{(a q)}^{-}...
Consider the reactions : 
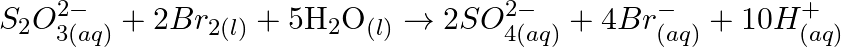 Why does the same reductant, thiosulphate react differently with iodine and bromine?
Why does the same reductant, thiosulphate react differently with iodine and bromine?
Solution: The normal oxidation no. of $\mathrm{S}$ in $\mathrm{S}_{2} \mathrm{O}_{3}^{2-}$ is $+2$. The normal oxidation no. of $\mathrm{S}$ in $S_{4} \mathrm{O}_{6}^{2-}$ is $+2.5$. The oxidation...
Identify the substance oxidised, reduced, oxidising agent and reducing agent for each of the following reactions: (a)  (b)
(b) ![Rendered by QuickLaTeX.com H C H O_{(l)}+2\left[A g\left(N H_{3}\right)_{2}\right]_{(a q)}^{+}+3 O H_{(a q)}^{-} \rightarrow 2 A g_{(s)}+H C O O_{(a q)}^{-}+](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-b8e4956dd6ca01b2ee5147606a038527_l3.png)
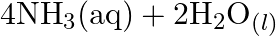 (c)
(c) 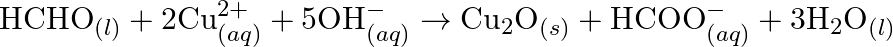 (d)
(d) 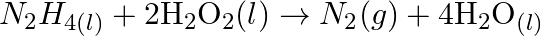 (e)
(e) 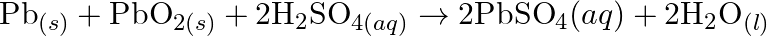
Solution: (a) $2 \mathrm{AgBr}_{(s)}+C_{6} H_{6} O_{2}(a q) \rightarrow 2 \mathrm{Ag}_{(s)}+2 \mathrm{HBr}_{(a q)}+C_{6} \mathrm{H}_{4} O_{2}(a q)$ $\mathrm{C}_{6} \mathrm{H}_{6}...
How do you count for the following observations? (a) Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene we use alcoholic potassium permanganate as an oxidant. Why? Write a balanced redox equation for the reaction. (b) When concentrated sulphuric acid is added to an inorganic mixture containing chloride, we get colourless pungent smelling gas HCl, but if the mixture contains bromide then we get red vapour of bromine. Why?
Solution: (a) While producing benzoic corrosive from toluene, alcoholic potassium permanganate is utilized as an oxidant because of the given reasons. (I) In an impartial medium, $O H^{-}$ions are...
Whenever a reaction between an oxidisina adent and a reducina aqent is carried out, a compound of lower oxidation state is formed if the reducing agent is in excess and a compound of higher oxidation state is formed if the oxidising agent is in excess. J ustify this statement giving three illustrations. Justify the above statement with three examples.
Solution: When there is a response between lessening specialist and oxidizing specialist, a compound is framed which has lower oxidation number if the diminishing specialist is in abundance and a...
The compound 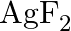 is unstable compound. However, if formed, the compound acts as a very strong oxidising agent. Why?
is unstable compound. However, if formed, the compound acts as a very strong oxidising agent. Why?
Solution: The oxidation no. of $A g$ in $A g F_{2}$ is $+2$. Be that as it may, $+2$ is entirely unsound oxidation no. of Ag. Consequently, when $A g F_{2}$ is framed, silver acknowledges an...
Consider the reactions: (a)  (b)
(b) 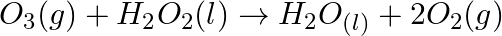 Why it is more appropriate to write these reactions as : (a)
Why it is more appropriate to write these reactions as : (a) 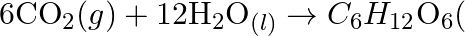 aq
aq 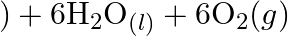 (b)
(b) 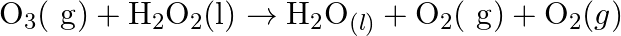 Also suggest a technique to investigate the path of the above (a) and (b) redox reactions
Also suggest a technique to investigate the path of the above (a) and (b) redox reactions
Solution: (a) Stage 1: $\mathrm{H}_{2} \mathrm{O}$ breaks to give $\mathrm{H}_{2}$ and $\mathrm{O}_{2}$. $2 \mathrm{H}_{2} \mathrm{O}_{(\mathrm{l})} \rightarrow 2...
While sulphur dioxide and hydrogen peroxide can act as oxidising as well as reducing agents in their reactions, ozone and nitric acid act only as oxidants. Why?
Solution: In sulfur dioxide $\left(S O_{2}\right)$ the oxidation no. of $\mathrm{S}$ is $+4$ and the scope of oxidation no. of sulfur is from $+6$ to $-2$. Consequently, $S O_{2}$ can go about as a...
Suggest a list of substances where carbon can exhibit oxidation states from -4 to +4 and nitrogen from -3 to +5.
Solution: The compound where carbon has oxidation number from -4 to +4 are given below in the table:
Write formulas for the following compounds: (a) Mercury (II) chloride (b) Nickel (II) sulphate (c) Tin (IV) oxide (d) Thallium (I) sulphate (e) Iron (III) sulphate (f) Chromium (III) oxide
Solution: Formulas are: (a) Mercury (II) chloride $H g C l_{2}$ (b) Nickel (II) sulphate $\mathrm{NiSO}_{4}$ (c) Tin (IV) oxide $\mathrm{SnO}_{2}$ (d) Thallium (I) sulphate $\mathrm{Tl}_{2}...
Calculate the oxidation number of sulphur, chromium and nitrogen in H2SO5, Cr2O2 and NOT. Suggest structure of these compounds. Count for the fallacy. nitrogen in H2SO5, Cr2O2 and NOT. Suggest structure of these compounds. Count for the fallacy.
Solution: O.N. of S in H2SO5. By traditional strategy, the O.N. of S in H2SO5 is 2 (+1) + x + 5 (- 2) = 0 or x = +8 This is outlandish on the grounds that the most extreme O.N. of S can't be more...
Fluorine reacts with ice and results in the change:  Justify that this reaction is a redox reaction
Justify that this reaction is a redox reaction
Solution: $H_{2} O_{(s)}+F_{2}(g) \rightarrow H F_{(g)}+H O F_{(g)}$ In the above response, Oxidation no. of $\mathrm{H}$ and $\mathrm{O}$ in $\mathrm{H}_{2} \mathrm{O}$ is $+1$ and $-2$...
Justify that the reactions is redox reactions: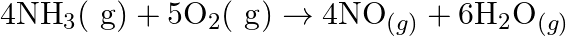
Solution: $4 \mathrm{NH}_{3(g)}+5 \mathrm{O}_{2}(g) \rightarrow 4 \mathrm{NO}_{(g)}+6 \mathrm{H}_{2} \mathrm{O}_{(g)}$ In the above response, Oxidation no. of $\mathrm{N}$ and $\mathrm{H}$ in $N...
Justify that the reactions is redox reactions: 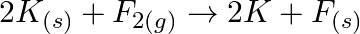
Solution: $2 K_{(s)}+F_{2(g)} \rightarrow 2 K+F_{(s)}$ In the above response, Oxidation no. of $\mathrm{K}$ is 0 . Oxidation no. of $F$ is 0 . Oxidation no. of $\mathrm{K}$ and $\mathrm{F}$ in...
Justify that the reactions is redox reactions: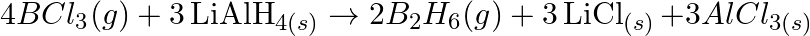
Solution: $4 \mathrm{BCl}_{3(g)}+3 \mathrm{LiAlH}_{4(s)} \rightarrow 2 \mathrm{~B}_{2} \mathrm{H}_{6}(\mathrm{~g})+3 \mathrm{LiCl}_{(s)}+3 \mathrm{AlCl}_{3}(s)$ the above response, Oxidation no. of...
Justify that the reactions is redox reactions: 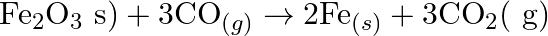
Solution: $\mathrm{Fe}_{2} \mathrm{O}_{3(s)}+3 \mathrm{CO}_{(g)} \rightarrow 2 \mathrm{Fe}_{(s)}+3 \mathrm{CO}_{2}(g)$ In the above response, Oxidation no. of Fe and $\mathrm{O}$ in $\mathrm{Fe}_{2}...
Justify that the reactions is redox reactions: 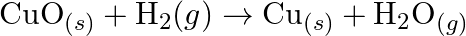
Solution: $\mathrm{CuO}_{(s)}+\mathrm{H}_{2}(\mathrm{~g}) \rightarrow \mathrm{Cu}_{(s)}+\mathrm{H}_{2} \mathrm{O}_{(g)}$ Oxidation no. of $\mathrm{Cu}$ and $\mathrm{O}$ in $C u O$ is $+2$ and $-2$...
What are the oxidation number of the underlined elements and how do you rationalise your results?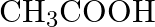
Solution: $\underline{\mathrm{C}} \mathrm{H}_{3} \underline{\mathrm{C}} \mathrm{OOH}$ C2x+14O2 \mathrm{C}_{2}^{\mathrm{x}}+1_{4} \mathrm{O}_{2} Let $x$ be the oxidation no. of $C$. Oxidation...
What are the oxidation number of the underlined elements and how do you rationalise your results?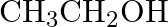
Solution: $\mathrm{CH}_{3} \mathrm{C} \mathrm{H}_{2} \mathrm{OH}$ $$ $$ Let $x$ be the oxidation no. of $C$. Oxidation no. of $\mathrm{O}=-2$ Oxidation no. of $\mathrm{H}=+1$ Then, at that...
What are the oxidation number of the underlined elements and how do you rationalise your results?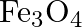
Solution: $\mathrm{Fe}_{3} \mathrm{O}_{4}$ Let $x$ be the oxidation no. of Fe. Oxidation no. of $0=-2$ Then, at that point, $3(x)+4(- 2)=0$ $3 x-8=0$ $x=\frac{8}{3}$ Oxidation no. can't be partial....
What are the oxidation number of the underlined element and how do you rationalise your results?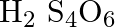
Solution: H2S4O6: Let x be the oxidation number of S. \[\begin{array}{*{35}{l}} Oxidation\text{ }no.\text{ }of\text{ }H\text{ }=\text{ }+1 \\ Oxidation\text{ }no.\text{ }of\text{ }O\text{ }=\text{...
What are the oxidation number of the underlined elements and how do you rationalise your results? 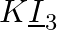
Solution: KI3: In KI3, the oxidation number (O.N.) of K is +1. Consequently, the normal oxidation number of I is Ncert Solutions Cbse Class 11-science Chemistry Chapter - Redox Reactions. In any...
Think about the components: Cs, Ne, I and F Identify the component that displays neither negative nor positive oxidation state?
Solution: Ne displays neither negative nor positive oxidation no. That is 0.
Think about the components: Cs, Ne, I and F Identify the component that displays both negative and positive oxidation states.
Solution: I displays both negative and positive oxidation no. That is - 1, +1, +3, +5 and +7.
Consider the elements: Cs, Ne, I and F Identify the element that exhibits only positive oxidation.
Solution: Cs displays just certain oxidation no. That is +1.
Think about the components: Cs, Ne, I and F Identify the component that displays just adverse oxidation.
Solution: F exhibits only negative oxidation number. That is -1.
Assign oxidation number to the underlined elements 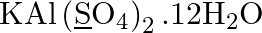
Solution: $\mathrm{KAl}\left(\underline{\mathrm{S}} \mathrm{O}_{4}\right)_{2} .12 \mathrm{H}_{2} \mathrm{O}$ Let expect oxidation number of S is x. \[\begin{array}{*{35}{l}}...
Assign the oxidation number to the underlined component 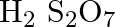
Solution: $\mathrm{H}_{2} \mathrm{~S}_{2} \mathrm{O}_{7}$ Let accept oxidation number of S is x. \[\begin{array}{*{35}{l}} Oxidation\text{ }number\text{ }of\text{ }O\text{ }=\text{...
Assign the oxidation number to the underlined component NaBH4
Solution: NaBH4 Let accept oxidation number of B is x. \[\begin{array}{*{35}{l}} Oxidation\text{ }number\text{ }of\text{ }Na\text{ }=\text{ }+1 \\ ~ \\ Oxidation\text{ }number\text{...
Assign the oxidation number to the underlined component CaO2
Solution: CaO2 Let expect oxidation number of O is x. \[Oxidation\text{ }number\text{ }of\text{ }Ca\text{ }=\text{ }+2\] Then, at that point, we have: \[\begin{array}{*{35}{l}}...
Assign the oxidation number to the underlined component K2MnO4
Solution: K2MnO4 Let expect oxidation number of Mn is x. \[\begin{array}{*{35}{l}} Oxidation\text{ }number\text{ }of\text{ }K\text{ }=\text{ }+1 \\ ~ \\ Oxidation\text{ }number\text{...
Assign the oxidation number to the underlined component H4P2O7
Solution: H4P2O7 Let expect oxidation number of P is x. \[\begin{array}{*{35}{l}} Oxidation\text{ }number\text{ }of\text{ }H\text{ }=\text{ }+1 \\ ~ \\ Oxidation\text{ }number\text{...
Assign the oxidation number to the underlined component NaHSO4
Solution: NaHSO4 Let expect oxidation number of S is x. \[\begin{array}{*{35}{l}} Oxidation\text{ }number\text{ }of\text{ }Na\text{ }=\text{ }+1 \\ ~ \\ Oxidation\text{ }number\text{...
Assign the oxidation number to the underlined component NaH2PO4
Solution: NaH2PO4 Let expect oxidation number of P is x. We realize that, \[\begin{array}{*{35}{l}} Oxidation\text{ }number\text{ }of\text{ }Na\text{ }=\text{ }+1 \\ ~ \\...
