Case 1: When $\varepsilon=0.1, \mathrm{R_O}=1 \mathrm{~A}$ $\mathrm R_1=8 \times 10^{-11}$ $\mathrm{M}=0.08 \mathrm{~nm}$ Velocity at ground level is given as $\mathrm v_1=1.44 \times 10^{6}...
The Bohr model for the H-atom relies on the Coulomb’s law of electrostatics. Coulomb’s law has not directly been verified for very short distances of the order of angstroms. Supposing Coulomb’s law between two opposite charge
The inverse square law in electrostatics is 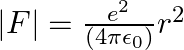 for the force between an electron and a proton. The
for the force between an electron and a proton. The 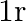 dependence of
dependence of 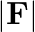 can be understood in quantum theory as being due to the fact that the ‘particle’ of light (photon) is massless. If photons had a mass
can be understood in quantum theory as being due to the fact that the ‘particle’ of light (photon) is massless. If photons had a mass 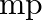 , force would be modified to
, force would be modified to ![Rendered by QuickLaTeX.com |F|=\frac{e^{2}}{\left(4 \pi \epsilon_{0}\right)} r^{2}\left[\frac{1}{r^{2}}+\frac{\lambda}{r}\right] \quad](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-514ad8afbf25cd57a0bc62f92316ce07_l3.png) where
where 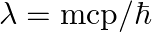 and
and 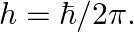 Estimate the change in the ground state energy of an H-atom if
Estimate the change in the ground state energy of an H-atom if 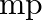 were
were 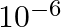 times the mass of an electron.
times the mass of an electron.
$m_{\mathrm{p}} c^{2}=10^{-6} \times$ electron mass $\times c^{2}$ $=10^{-6} \times 0.5 \mathrm{MeV}$ $\approx 10^{-6} \times 0.5 \times 1.6 \times 10^{-13}$ $\approx \mathrm{O} .8 \times 1...
In the Auger process, an atom makes a transition to a lower state without emitting a photon. The excess energy is transferred to an outer electron which may be ejected by the atom. (This is called an Auger electron). Assuming the nucleus to be massive, calculate the kinetic energy of an 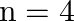 Auger electron emitted by Chromium by absorbing the energy from an
Auger electron emitted by Chromium by absorbing the energy from an 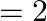 to
to 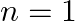 transition.
transition.
The energy in the nth state can be calculated as, $-\operatorname{Rch} Z^{2} / n^{2}=-13.6 z^{2} / n^{2} e V$ Where $R=$ Rydberg constant and $\mathrm{Z}=24$ Energy released is given as $\left(13.6...
Deuterium was discovered in 1932 by Harold Urey by measuring the small change in wavelength for a particular transition in 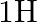 and
and 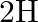 . This is because the wavelength of transition depends to a certain extent on the nuclear mass. If nuclear motion is taken into account then the electrons and nucleus revolve around their common centre of mass. Such a system is equivalent to a single particle with a reduced mass
. This is because the wavelength of transition depends to a certain extent on the nuclear mass. If nuclear motion is taken into account then the electrons and nucleus revolve around their common centre of mass. Such a system is equivalent to a single particle with a reduced mass 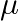 , revolving around the nucleus at a distance equal to the electron-nucleus separation. Here
, revolving around the nucleus at a distance equal to the electron-nucleus separation. Here 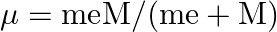 where
where 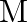 is the nuclear mass and
is the nuclear mass and 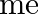 is the electronic mass. Estimate the percentage difference in wavelength for the 1 st line of the Lyman series in
is the electronic mass. Estimate the percentage difference in wavelength for the 1 st line of the Lyman series in 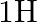 and
and 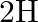 . (Mass of
. (Mass of 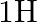 nucleus is
nucleus is 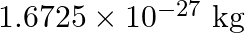 , Mass of
, Mass of 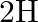 nucleus is
nucleus is 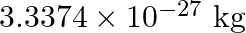 , Mass of electron
, Mass of electron 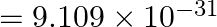 kg.)
kg.)
The energy of an electron in the nth state is given by the expression, $E_ n=-\mu Z^{2} \mathrm{e}^{4} / 8 \varepsilon_ 0^{2} h^{2}\left(1 / n^{2}\right)$ For hydrogen atom we have, $\mu...
The first four spectral lines in the Lyman series of an H-atom are 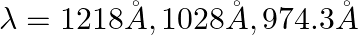 and
and 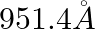 . If instead of Hydrogen, we consider Deuterium, calculate the shift in the wavelength of these lines.
. If instead of Hydrogen, we consider Deuterium, calculate the shift in the wavelength of these lines.
Let the reduced masses of electrons of hydrogen and deuteriur be $\mu _{H}$ and $\mu _{D}$ The fixed mass series for hydrogen and deuterium be $n_i$ and $n_f$ $R_h / R_d=\mu _H / \mu _D$ Reduced...
What is the minimum energy that must be given to an 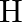 atom in ground state so that it can emit an Hy line in Balmer series? If the angular momentum of the system is conserved, what would be the angular momentum of such Hy photon?
atom in ground state so that it can emit an Hy line in Balmer series? If the angular momentum of the system is conserved, what would be the angular momentum of such Hy photon?
Energy required for the transition from $n=1$ to $n=5$ can be calculated as, $E=E _5-E_ 1=\left(-13.6 / 5^{2}\right)-\left(-13.6 / 1^{2}\right)=13.06 \mathrm{eV}$ Angular momentum is given as change...
Show that the first few frequencies of light that are emitted when electrons fall to the 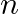 th level from levels higher than
th level from levels higher than 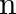 are approximate harmonics (i.e. in the ratio
are approximate harmonics (i.e. in the ratio 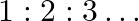 ) when
) when 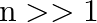 .
.
The difference between the two atoms is used to indicate the frequency of any line in the hydrogen spectrum series. $\mathrm{f_{cm}}=\mathrm{cRZ}^{2}\left[1 /(\mathrm{n}+\mathrm{p})^{2}-1 /...
Using the Bohr model, calculate the electric current created by the electron when the H-atom is in the ground state.
Let the velocity of the electron be $v$ Number of revolutions per unit time is given as $\mathrm{f}=2 \mathrm{ma}_{0} / \mathrm{V}$ The electric current is given as $I=Q$ When change $Q$ flows in...
Assume that there is no repulsive force between the electrons in an atom but the force between positive and negative charges is given by Coulomb’s law as usual. Under such circumstances, calculate the ground state energy of a He-atom.
For He nucleus we have, $Z=2$ and in ground state, $n=1$ As a result, $E n=-13.6 Z^{2} / n^{2} e V=-54.4 \mathrm{eV}$
Positronium is just like an H-atom with the proton replaced by the positively charged anti-particle of the electron (called the positron which is as massive as the electron). What would be the ground state energy of positronium?
The positronium has the lowest energy of -6.8 electron volts. The greatest energy level of positronium is -1.7 electron volt, which is the next highest energy level after n = 1.
Consider two different hydrogen atoms. The electron in each atom is in an excited state. Is it possible for the electrons to have different energies but the same orbital angular momentum according to the Bohr model?
Because the value of n in two hydrogen atoms differs, their angular momentum differs as well. The angular momentum, according to Bohr's model, is given as L = nh/2π
The mass of H atom is less than sum of the masses of a proton and electron. Why is this?
Protons and neutrons (and other particles) are held together by something called the strong nuclear force (strong interaction). This force diminishes the system's overall energy, which is dissipated...
Let 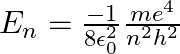 be the energy of the nth level of H-atom. If all the H-atoms are in the ground state and radiation of frequency
be the energy of the nth level of H-atom. If all the H-atoms are in the ground state and radiation of frequency 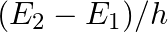 falls on it
falls on it
(a) it will not be absorbed at all
(b) some of the atoms will move to the first excited state
(c) all atoms will be excited to the 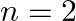 state
state
(d) no atoms will make a transition to the 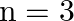 state
state
The correct options are: (b) some of the atoms will move to the first excited state (d) no atoms will make a transition to the $n=3$ state
The Balmer series for the H-atom can be observed
(a) if we measure the frequencies of light emitted when an excited atom falls to the ground state
(b) if we measure the frequencies of light emitted due to transitions between excited states and the first excited state
(c) in any transition in a 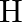 -atom
-atom
(d) as a sequence of frequencies with the higher frequencies getting closely packed
The correct options are: (b) if we measure the frequencies of light emitted due to transitions between excited states and the first excited state (d) as a sequence of frequencies with the higher...
The Bohr model for the spectra of an H-atom
(a) will not be applicable to hydrogen in the molecular from
(b) will not be applicable as it is for a He-atom
(c) is valid only at room temperature
(d) predicts continuous as well as discrete spectral lines
The correct options are: (a) will not be applicable to hydrogen in the molecular from (b) will not be applicable as it is for a $\mathrm{He}$-atom
Consider aiming a beam of free electrons towards free protons. When they scatter, an electron and a proton cannot combine to produce an H-atom,
(a) because of energy conservation
(b) without simultaneously releasing energy in the form of radiation
(c) because of momentum conservation
(d) because of angular momentum conservation
The correct options are: (a) because of energy conservation (b) without simultaneously releasing energy in the form of radiation
An ionised H-molecule consists of an electron and two protons. The protons are separated by a small distance of the order of angstrom. In the ground state,
(a) the electron would not move in circular orbits
(b) the energy would be (2) 4 times that of an 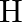 -atom
-atom
(c) the electrons, the orbit would go around the protons
(d) the molecule will soon decay in a proton and an 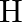 -atom
-atom
The correct options are: (a) the electron would not move in circular orbits (c) the electrons, the orbit would go around the protons
A set of atoms in an excited state decays.
(a) in general to any of the states with lower energy
(b) into a lower state only when excited by an external electric field
(c) all together simultaneously into a lower state
(d) to emit photons only when they collide
The correct option is: (a) in general to any of the states with lower energy
Two H atoms in the ground state collide inelastically. The maximum amount by which their combined kinetic energy is reduced is
(a) 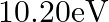
(b) 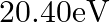
(c) 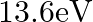
(d) 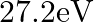
The correct option is: (a) $10.20 \mathrm{eV}$
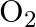 molecule consists of two oxygen atoms. In the molecule, nuclear force between the nuclei of the two atoms
molecule consists of two oxygen atoms. In the molecule, nuclear force between the nuclei of the two atoms
(a) is not important because nuclear forces are short-ranged
(b) is as important as an electrostatic force for binding the two atoms
(c) cancels the repulsive electrostatic force between the nuclei
(d) is not important because the oxygen nucleus have an equal number of neutrons and protons
The correct option is: (a) is not important because nuclear forces are short-ranged
For the ground state, the electron in the H-atom has an angular momentum 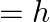 , according to the simple Bohr model. Angular momentum is a vector and hence there will be infinitely many orbits with the vector pointing in all possible directions. In actuality, this is not true,
, according to the simple Bohr model. Angular momentum is a vector and hence there will be infinitely many orbits with the vector pointing in all possible directions. In actuality, this is not true,
(a) because Bohr model gives incorrect values of angular momentum
(b) because only one of these would have a minimum energy
(c) angular momentum must be in the direction of spin of electron
(d) because electrons go around only in horizontal orbits
The correct option is: (a) because Bohr model gives incorrect values of angular momentum
The simple Bohr model cannot be directly applied to calculate the energy levels of an atom with many electrons. This is because
(a) of the electrons not being subject to a central force
(b) of the electrons colliding with each other
(c) of screening effects
(d) the force between the nucleus and an electron will no longer be given by Coulomb’s law
The correct option is: (a) of the electrons not being subject to a central force
The binding energy of an H-atom, considering an electron moving around a fixed nucleus (proton), is 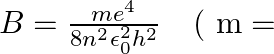 electron mass
electron mass 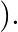 If one decides to work in a frame of reference where the electron is at rest, the proton would be moving around it. By similar arguments, the binding energy would be
If one decides to work in a frame of reference where the electron is at rest, the proton would be moving around it. By similar arguments, the binding energy would be 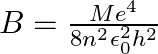 (M = proton mass) This last expression is not correct because
(M = proton mass) This last expression is not correct because
(a) n would not be integral
(b) Bohr-quantisation applies only to electron
(c) the frame in which the electron is at rest is not inertial
(d) the motion of the proton would not be in circular orbits, even approximately
The correct option is: (c) the frame in which the electron is at rest is not inertial
Taking the Bohr radius as 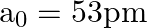 , the radius of
, the radius of 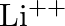 ion in its ground state, on the basis of Bohr’s model, will be about
ion in its ground state, on the basis of Bohr’s model, will be about
(a) 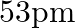
(b) 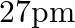
(c) 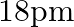
(d) 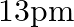
The correct option is: (c) $18 \mathrm{pm}$
Obtain the first Bohr’s radius and the ground state energy of a muonic hydrogen atom [i.e., an atom in which a negatively charged muon (μ−) of mass about 207me orbits around a proton].
Answer – We are given that the mass of a negatively charged muon is mμ = 207 me Now, According to Bohr’s model bohr’s radius is given by – \[{{r}_{e}}\alpha \left( \frac{1}{{{m}_{e}}}...
If Bohr’s quantization postulate (angular momentum = nh/2π) is a basic law of nature, it should be equally valid for the case of planetary motion as well. Why then do we never speak of quantization of orbits of planets around the sun?
Answer – The quantum level for a planetary motion is taken to be continuous. This can be explained by the fact that the planetary motion’s angular momentum is largely relative to the value of...
The total energy of an electron in the first excited state of the hydrogen atom is about –3.4 eV.
(a) What is the kinetic energy of the electron in this state?
(b) What is the potential energy of the electron in this state?
(c) Which of the answers above would change if the choice of the zero of potential energy is changed?
Answer – (a) Total energy of the electron is given by E = – 3.4 eV We know that the kinetic energy of the electron is equal to the negative of the total energy. So, K.E = – E K.E = – (- 3.4 ) = +...
Classically, an electron can be in any orbit around the nucleus of an atom. Then what determines the typical atomic size? Why is an atom not, say, thousand times bigger than its typical size? The question had greatly puzzled Bohr before he arrived at his famous model of the atom that you have learnt in the text. To simulate what he might well have done before his discovery, let us play as follows with the basic constants of nature and see if we can get a quantity with the dimensions of length that is roughly equal to the known size of an atom (~ 10–10m).
(a) Construct a quantity with the dimensions of length from the fundamental constants e, me , and c. Determine its numerical value.(b) You will find that the length obtained in (a) is many...
Obtain an expression for the frequency of radiation emitted when a hydrogen atom de-excites from level n to level (n–1). For large n, show that this frequency equals the classical frequency of revolution of the electron in the orbit.
Answer – We are given that the hydrogen atom de-excites from the level n to level (n-1). Now, writing the equation for energy (E1) of the radiation for the level n. we have –...
The gravitational attraction amongst proton and electron in a hydrogen atom is weaker than the coulomb attraction by a component of around 10−40. Another option method for taking a gander at this case is to assess the span of the first Bohr circle of a hydrogen particle if the electron and proton were bound by gravitational attraction. You will discover the appropriate response fascinating.
Answer – We know that the radius of the first Bohr orbit is given by the relation – \[{{r}_{1}}=\frac{4\pi {{\in }_{0}}{{\left( \frac{h}{2\pi } \right)}^{^{2}}}}{{{m}_{e}}{{e}^{2}}}\] Where,...
Choose a suitable solution to the given statements which justify the difference between Thomson’s model and Rutherford’s model
(c) For a small thickness T, keeping other factors constant. It has been found that amount of alpha particles scattered at direct angles is proportional to T. This linear dependence implies? (d) To...
Choose a suitable solution to the given statements which justify the difference between Thomson’s model and Rutherford’s model
(a) In the case of scattering of alpha particles by a gold foil. Average angle of deflection of alpha particles stated by Rutherford’s model is (less than, almost the same as, much greater than)...
In accordance with the Bohr’s model, find the quantum number that characterizes the earth’s revolution around the sun in an orbit of radius 3 × 1011 m with orbital speed 3 × 104 m/s. (Mass of earth = 6.0 × 1024 kg.)
Answer – We know that the radius of the Earth’s orbit around the Sun is given by r = 1.5 × 1011 m We are given the orbital speed of the Earth, ν = 3 × 104 m/s Also, the mass of the Earth,...
A 12.5 eV electron beam is used to bombard gaseous hydrogen at room temperature. What series of wavelengths will be emitted?
Answer – It has been given that 12.5 eV is the energy of the electron beam used to bombard gaseous hydrogen at room temperature. Also, −13.6 eV is the ground state energy of the gaseous...
The radius of the innermost electron orbit of a hydrogen atom is 5.3×10–11 m. What are the radii of the n = 2 and n =3 orbits?
Answer – We are given the radius of the innermost orbit of a hydrogen atom as r1 = 5.3 × 10−11 m. Suppose r2 represents the radius of the orbit for the level n = 2. Then, It will...
Calculate the orbital period in each of these levels.
The speed of the electron for n=1, n=2, and n=3 is 2.18×106m/s, 1.09×106m/s and 7.27 × 105 m/s in a hydrogen atom. Answer - Suppose, T1 represents the orbital period of the electron when it is...
Using the Bohr’s model, calculate the speed of the electron in a hydrogen atom in the n = 1, 2, and 3 levels.
Answer – Let the orbital speed of the electron in the ground state level of an hydrogen atom, n1= 1, be given by v1 . For charge (e) of an electron, v1 is given by the relation –...
A hydrogen atom initially in the ground level absorbs a photon, which excites it to the n = 4 level. Determine the wavelength and frequency of photon.
Answer – We know that for ground level, n1 = 1 Suppose E1 is the energy of the level n1. So as we know E1 is related with n1 as – \[{{E}_{1}}=\frac{13.6}{{{n}_{1}}^{2}}eV=-13.6eV\]...
The ground state energy of hydrogen atom is –13.6 eV. What are the kinetic and potential energies of the electron in this state?
Ans: We know that the ground state energy of hydrogen atom is given by – E = − 13.6 eV The total energy of hydrogen atom is equal to -13.6 eV. It is understood that the kinetic energy is equal to...
A difference of 2.3 eV separates two energy levels in an atom. What is the frequency of radiation emitted when the atom make a transition from the upper level to the lower level?
Answer – We know that the separation of two energy levels in an atom is given by the relation – \[E=2.3eV=2.3\times 1.6\times {{10}^{-19}}\] \[E=3.68\times {{10}^{-19}}J\] Let v be the frequency of...
What is the shortest wavelength present in the Paschen series of spectral lines?
Answer – Using the Rydberg’s formula, given the relation – \[\frac{hc}{\lambda }=21.76\times {{10}^{-19}}[\frac{1}{{{n}_{1}}^{2}}-\frac{1}{{{n}_{2}}^{2}}]\] Where h is the Planck’s constant, given =...
Suppose you are given a chance to repeat the alpha-particle scattering experiment using a thin sheet of solid hydrogen in place of the gold foil. (Hydrogen is a solid at temperatures below 14 K.) What results do you expect?
Answer – It is quite known that the mass of the incident alpha particle (6.64 × 10-27kg) is much more than the mass of hydrogen (1.67 × 10-27Kg). Hence, the alpha particle would fail to rebound...
Choose the correct alternative from the clues given at the end of each statement:
(d) An atom shows continuous mass distribution in a ……….but a very high non-uniform mass distribution in ……….(Thomson’s model/ Rutherford’s model.) (e) In ………. the positively charged part of the...
Choose the correct alternative from the clues given at the end of each statement:
(a) In Rutherford’s model the atomic size is ……….. the size of the atom in Thomson’s model. (much greater than/no different from/much less than.) (b) ) In ………. the ground state electrons...
Obtain the first Bohr’s radius and the ground state energy of a muonic hydrogen atom [i.e., an atom in which a negatively charged muon (μ−) of mass about 207me orbits around a proton].
Answer – We are given that the mass of a negatively charged muon is mμ = 207 me Now, According to Bohr’s model bohr’s radius is given by – \[{{r}_{e}}\alpha \left( \frac{1}{{{m}_{e}}}...
If Bohr’s quantization postulate (angular momentum = nh/2π) is a basic law of nature, it should be equally valid for the case of planetary motion as well. Why then do we never speak of quantization of orbits of planets around the sun?
Answer – The quantum level for a planetary motion is taken to be continuous. This can be explained by the fact that the planetary motion’s angular momentum is largely relative to the value of...

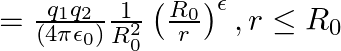 Calculate in such a case, the ground state energy of an
Calculate in such a case, the ground state energy of an