Answer: At the cathode, greyish white metal lead is created, whilst at the anode, dark reddish-brown gases are formed.
Name the kind of particles present in
a. Sodium hydroxide solution
b. Carbonic acid
c. Sugar solution
Solution: a. Na+ ions and OH– ions b. H+, HCO3, CO32- ions and H2CO3 molecules c. C12H22O11 molecules
M2O is the oxide of metal “M” which is above hydrogen in the activity series. M2O, when dissolved in water, forms the corresponding hydroxide which is a good conductor of electricity.
a. State the reaction taking place at the cathode.
b. Name the product at the anode.
Solution: Answer: i)M+ + 1e– → M ii) Oxygen gas is produced at the anode. Explanation: i) The activity series gives us a metal oxide M2O of metal ‘M'. M2O dissolves in water to generate hydroxide...
Given reasons why: c) Although copper is a good conductor of electricity, it is a non-electrolyte.
Answer: Copper is a metal that contains free electrons. Electricity is conducted through the movement of free electrons. Copper, on the other hand, does not dissolve into ions. As a result, it does...
Give reasons why:
a. Sodium chloride conducts electricity only infused or aqueous solution state.
b. In the electroplating of an article with silver, the electrolyte sodium Argento cyanide solution is preferred over the silver nitrate solution.
Solution: a) In the solid-state, the electrostatic forces of attraction between molecules are extremely strong, attracting molecules to one another. In the fused state, these forces grow weaker, and...
State which electrode: anode or cathode is an oxidizing electrode? Give a reason for the same.
Answer: During the oxidation process, electrons are released, and the anode has a sufficient concentration of electrons to allow for the release of electrons. As a result, the anode serves as the...
Give one word for Electrolytic deposition of a superior metal on a baser metal.
Answer: Galvanisation Explanation: Anodizing is the process of electroplating. Electroplating is the technique of plating a metal on another metal using hydrolysis.
Identify a gas that cannot conduct electricity in the liquid state but conducts electricity when dissolved in water.
Answer: Hydrogen chloride gas does not conduct electricity when dissolved in water. In a diluted state, electrons can flow freely. Thus, it can conduct electricity and extract hydrogen from acids....
State which of these will act as a non-electrolyte:
a) Liquid carbon tetrachloride
b) Acetic acid
c) Sodium hydroxide aqueous solution
d) Potassium chloride aqueous solution
Solution: Non-electrolytes, on the other hand, are notoriously poor conductors of electricity. In addition, the solutions of these non-electrolytes do not transmit electrical currents. Liquid carbon...
Give reason: An aqueous solution of sodium chloride conducts electricity.
Answer: An aqueous solution of sodium chloride conducts electricity because it is a soluble ionic compound that, when dissolved in water, dissociates into two ions: Na ion and Cl ion, both of which...
Match the columns:
Answer: Ammonium hydroxide solution - This solution contains both ions and molecules of ammonium....
Differentiate between the electrical conductivity of copper sulfate solution and that of copper metal.
Answer:
Give reason: Electrolysis of acidulated water is considered to be an example of electrolysis.
Answer: It is believed to be an example of catalysis since pure water is not a good conductor of electricity, but when acid is introduced it catalyzes the ionization of water, and water is...
During electroplating of an article with nickel,
(i) Name
a. The electrolyte
b. The cathode
c. The anode
(ii) Give the reaction of electrolysis at
a. The cathode
b. The anode
Solution: (i) a. Nickel sulfate b. Article c. Pure nickel plate (ii) a. Ni2+ + 2e– → Ni b. Ni – 2e– → ...
The aqueous solution of Nickle sulphate contains
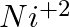 and
and 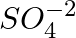 ions.
ions.
1. Which ion moves towards cathode?
2. What is the product at the anode?
Solution: Ni2+ ions move towards the cathode. If Reaction At anode: (with nickel electrodes) As a result, nickel dissolves from the anode and is replaced by nickel ions. If platinum...
Correct the given statement: “Lead bromide conducts electricity.”
Electrolysis of molten Lead bromide,The reaction that takes place at the anode:$$2 Br ^{-} \rightarrow Br _{2}$$Reaction that takes place at cathode:$$Pb ^{+2}+2 e ^{-} \rightarrow Pb$$Lead bromide...
Select the right answer. The aqueous solution of the compound which contains both ions and molecules is:
a. 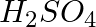 b) HCl
b) HCl
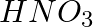 d)
d) 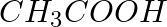
Solution:Answer is option d) Due to the fact that acetic acid is a good electrolyte, it dissociates very little and generates few ions, resulting in a solution that contains both molecules and ions....
A strip of copper is placed in four different colorless salt solutions. They are 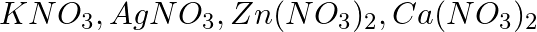 . Which of the solutions will finally turn blue?
. Which of the solutions will finally turn blue?
Answer: The colour of AgNO3 changes to blue. This is due to copper's higher position in the electrochemical series, which causes it to displace silver and generate Cu(NO3)2, which is blue in...
Write two applications of electrolysis in which anode diminishes in mass.
Solution: Essentially, a redox reaction is a chemical event in which electrons are transferred between the two reactants that are involved in the reaction. The migration of electrons can be...
Element X is a metal with valency 2. Element Y is a non-metal with valency 3.
1. Write equations to show how X and Y form ions.
2. If Y is a diatomic gas, write an equation for the direct combination of X and Y to form a compound.
If the compound formed between X and Y is melted and an electric current is passed through the molten compound, element X will be obtained at the ________ and Y at the ________ of the electrolytic cell.
Solution: X → X2+ + 2e– ; Y + 3e– → Y3-Y2 + 3X → X3 Y2Cathode, anode
Copy and complete the following table which shows two practical applications of electrolysis.
Solution:
Give reasons for the following:
k) Potassium is not extracted by electrolysis of its aqueous salt solution.
Answer: Potassium reacts with water in a variety of ways. Therefore, electrolysis of its aqueous salt solution is ruled out as an alternative method of production.
Give reasons for the following:
i) For electroplating with silver, silver nitrate is not used as an electrolyte.
j) Carbon tetrachloride is a liquid but does not conduct electricity.
Solution: i)...
Give reasons for the following: g) Ammonia is unionized in the gaseous state but in the aqueous solution, it is a weak electrolyte.
h) A graphite anode is preferred to other inert electrodes during electrolysis of fused lead bromide.
Answer: g) Because ammonia is a covalent molecule, it is unionized in the gaseous state, but it produces $NH_4OH$ in an aqueous solution, which can dissociate into ions when exposed to water. h)...
Give reasons for the following: e) The ratio of hydrogen and oxygen formed at the cathode and anode is 2:1 by volume.
f) In the electrolysis of acidified water dilute sulphuric acid is preferred to dilute nitric acid for acidification.
Answer: e) In the electrolytic reactions, 4H1+ is required at the cathode and 4OH...
Give reasons for the following:
c) Lead bromide undergoes electrolytic dissociation in the molten state, but it is a non-electrolyte in the solid state.
d) Aluminium is extracted from its oxide by electrolytic reduction and not by conventional reducing agents.
Answer: c) During the melting process of lead bromide, the ions are free and loosely packed. It is because of the electrostatic force of attraction that the ions are closely packed in the...
Give reasons for the following:
a) Electrolysis of molten lead bromide is a reaction in which oxidation and reduction go side by side, i.e., a redox reaction.
b) The blue color of aqueous copper sulfate fades when it is electrolyzed using platinum electrodes.
Solution: a) Bromine loses electrons at the anode of lead bromide electrolysis, while lead gets electrons at the cathode of lead bromide electrolysis. As a result, it is classified as a redox...
