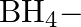Solution:
(I) 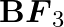
Boron will in general shape monomeric covalent halides in view of its little size and high electronegativity. These halides of boron generally have a planar three-sided calculation, which can be clarified by the cross-over of three  hybridized orbitals of boron with the sp orbitals of 3 halogen particles. It very well may be noticed that boron is
hybridized orbitals of boron with the sp orbitals of 3 halogen particles. It very well may be noticed that boron is  hybridized in
hybridized in 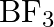 .
.


(ii)