Solution:
(I) 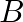 to
to 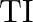
Gathering 13 components have their electronic design of 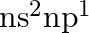 and the oxidation state displayed by these components ought to be 3 . Aside from these two electrons boron and aluminum, different components of this gathering show both
and the oxidation state displayed by these components ought to be 3 . Aside from these two electrons boron and aluminum, different components of this gathering show both 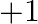 and
and 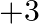 oxidation states. Boron and aluminum show oxidation condition of
oxidation states. Boron and aluminum show oxidation condition of 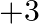 because of the idle pair impact. The two electrons, which are available in the S-shell don’t take part in holding as they are emphatically drawn in by the core. As we drop down the gathering, the latent pair impact turns out to be more noticeable. Subsequently, Ga
because of the idle pair impact. The two electrons, which are available in the S-shell don’t take part in holding as they are emphatically drawn in by the core. As we drop down the gathering, the latent pair impact turns out to be more noticeable. Subsequently, Ga 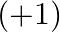 is unsteady and
is unsteady and 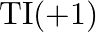 is entirely steady On dropping down the gathering, the steadiness of the
is entirely steady On dropping down the gathering, the steadiness of the 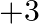 oxidation state gets diminished.
oxidation state gets diminished.

(ii) 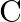 to
to 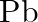
The electronic design of gathering 14 components is 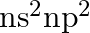 . Thus, the most well-known oxidation state showed by them ought to be
. Thus, the most well-known oxidation state showed by them ought to be 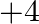 . After dropping down the gathering, the
. After dropping down the gathering, the 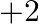 oxidation state turns out to be progressively normal and the moderately higher oxidation states become less steady. This is a direct result of the idle pair impact. Si and
oxidation state turns out to be progressively normal and the moderately higher oxidation states become less steady. This is a direct result of the idle pair impact. Si and  generally show the
generally show the 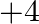 state. Despite the fact that
state. Despite the fact that 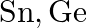 and
and 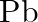 show the
show the 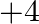 and
and 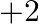 states, the security of higher oxidation states decline while dropping down the gathering.
states, the security of higher oxidation states decline while dropping down the gathering.

Security of 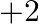 state increments
state increments
Ci Se Sn Pb
Security of 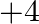 state diminishes
state diminishes
