Solution:
(b) Group 14 components have 4 valence electrons. Thus, bunch oxidation status is 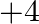 . In any case, the lower oxidation state turns out to be progressively steady because of the inactive pair impact, and the higher oxidation state turns out to be less steady.
. In any case, the lower oxidation state turns out to be progressively steady because of the inactive pair impact, and the higher oxidation state turns out to be less steady.
Accordingly, this gathering shows 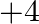 and
and 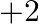 oxidation states.
oxidation states.

