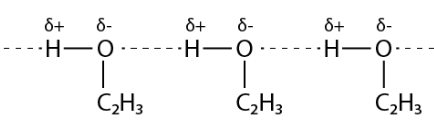
Propanol undergoes intermolecular H-bonding due to the presence of the – OH group. Butane, on the other hand, does not have the same advantages.
In order to dissolve the intermolecular hydrogen bonds, more energy would be necessary.
The hydrocarbon butane has a lower boiling point than propanol because of this.