Given,
V = 100 ml
V = 0.1 litre
![]()
![]()
![]()
Find out osmotic pressure of glucose solutions of 5% sol at 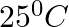 while molecular mass of glucose 180 and R = 0.0821 litre atmosphere.
while molecular mass of glucose 180 and R = 0.0821 litre atmosphere.
Find out osmotic pressure of glucose solutions of 5% sol at 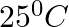 while molecular mass of glucose 180 and R = 0.0821 litre atmosphere.
while molecular mass of glucose 180 and R = 0.0821 litre atmosphere.
