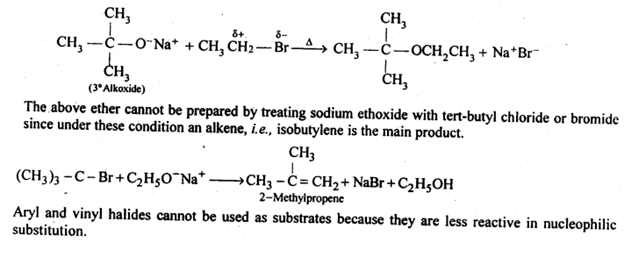
Williamson’s synthesis is a flexible approach for making symmetrical and unsymmetrical ethers. However, careful reactant selection is required for the synthesis of unsymmetrical ethers. Because the SN2 mechanism is used in Williamson’s synthesis and primary alkyl halides are the most reactive in the Sn2 reaction, the greatest yields of unsymmetrical ethers are achieved when the alkyl halides are primary and the alkoxide is primary, secondary, or tertiary. Tert-butylethyl ether, for example, is made by reacting ethyl bromide with sodium tert-butoxide.