Solution:
(a) Sodium can be extricated from sodium chloride by Downs measure.
This interaction includes the electrolysis of intertwined NaCl (40%) and CaCl2 (60 %) at a temperature of 1123 K in Downs cell.
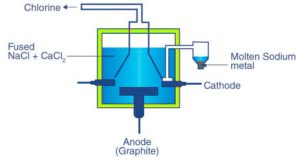
Steel is the cathode and a square of graphite goes about as the anode. Metallic Na and Ca are shaped at cathode. Liquid sodium is removed from the cell and gathered over lamp fuel.

b) Sodium hydroxide can be ready by the electrolysis of sodium chloride. This is called Castner-Kellner measure. In this interaction, the salt water arrangement is electrolysed utilizing a carbon anode and a mercury cathode.
The sodium metal, which is released at cathode, joins with mercury to shape a combination.

(c) Sodium peroxide First, NaCl is electrolysed to bring about the development of Na metal (Downs measure). This sodium metal is then warmed on aluminum plate in air (liberated from CO2) to shape its peroxide.
![]()
(d) Sodium carbonate is ready by Solvay measure. Sodium hydrogen carbonate is accelerated in a response of sodium chloride and ammonium hydrogen carbonate.
![Rendered by QuickLaTeX.com \[\begin{array}{*{35}{l}} 2NH3\text{ }+\text{ }H2O\text{ }+\text{ }CO2\text{ }\to \text{ }\left( NH4 \right)2CO3 \\ ~ \\ \left( NH4 \right)2CO3\text{ }+\text{ }H2O\text{ }+\text{ }CO2\text{ }\to \text{ }2NH4HCO3 \\ ~ \\ NH4HCO3\text{ }+\text{ }NaCl\text{ }\to \text{ }NH4Cl\text{ }+\text{ }NaHCO3 \\ \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-e76ffffa60b89ca8752eb140ba4ceb1b_l3.png)
These sodium hydrogen carbonate gems are warmed to give sodium carbonate.
![]()
