Solution:
(a) Borax is warmed firmly
Borax goes through different changes when warmed. It is losing atoms and expands of water right away. Then, at that point, it turns into a clear fluid, which sets to shape a glass-like material called a borax globule.
(b) Boric corrosive is added to water
At the point when boric corrosive is added to water, it acknowledges electrons from – OH particle.
(c) Aluminum is treated with weaken NaOH
Aluminum responds to sodium tetrahydroxoaluminate(III) by framing weaken NaOH. In this cycle, hydrogen gas is freed.
(d) 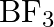 is responded with alkali
is responded with alkali
