Solution:
(a) Catenation is the property of carbon atoms self-combining to create a lengthy chain. Catenation is a characteristic that exists between carbon and silicon.
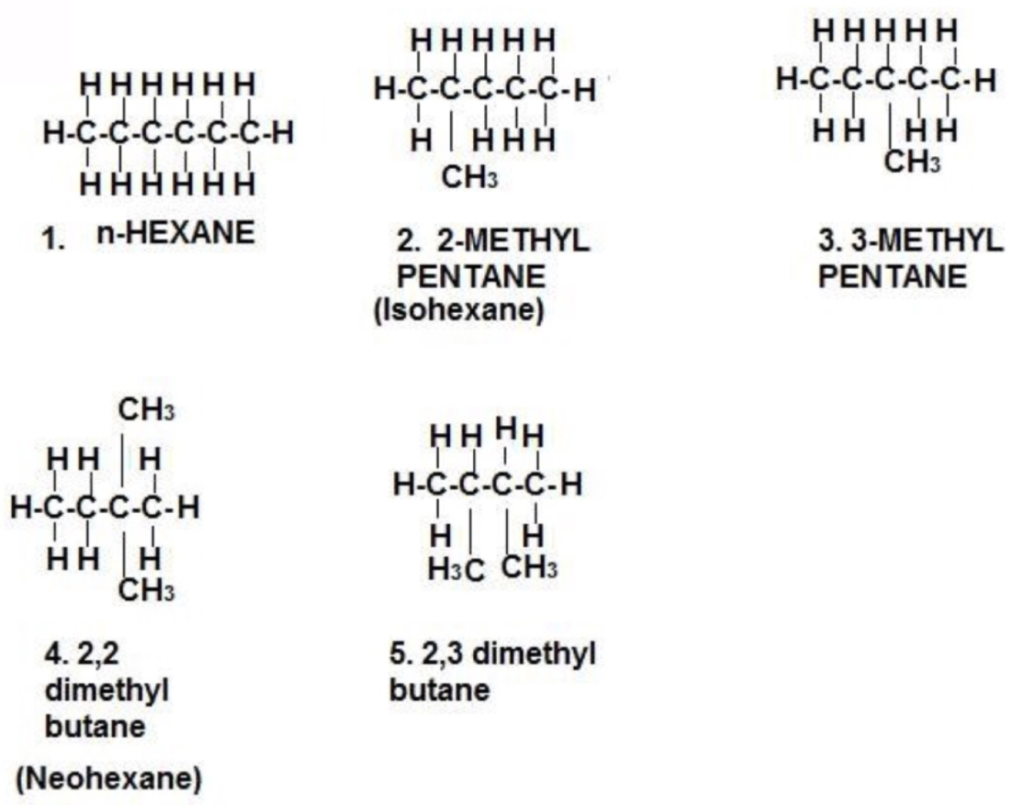
(b) Hexane has five isomers:
- Hexane, CH3CH2CH2CH2CH2CH3, a straight chain of six carbon atoms.
- 2-Methylpentane (Isohexane), CH3CH(CH3)CH2CH2CH3, a five-carbon chain with one methyl branch on the second.
- 3-Methylpentane, CH3CH2CH(CH3)CH2CH3, a five-carbon chain with one methyl branch on the third.
- 2,3-Dimethylbutane, CH3CH(CH3)CH(CH3)CH3, a four-carbon chain with one methyl branch on the second and third.
- 2,2-Dimethylbutane (neohexane), CH3C(CH3)2CH2CH3, a four-carbon chain with two methyl branches on the second.