Correct option is C fluorine
Florine is the most powerful oxidizing agent because it is the most electronegative element. Electronegative elements have the property to remove electrons form the elements to which, it is attached.
Which one of the following hologens is the strongest oxidising agent?
(A) I
(B)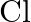
(C) F
(D)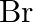
(A) I
(B)
(C) F
(D)
Which one of the following hologens is the strongest oxidising agent?
(A) I
(B)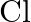
(C) F
(D)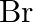
(A) I
(B)
(C) F
(D)
