Solution:

The number of carbon, hydrogen, and oxygen atoms in an organic compound can thus be expressed as:

Therefore, the empirical formula of the compound is C5H10O. Now, the empirical formula mass of the compound can be given as:

Molecular mass of the compound = 86
Therefore, the molecular formula of the compound is given by C5H10O.
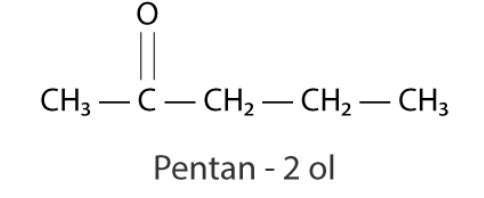
Because the provided compound does not reduce Tollen’s reagent, it is not an aldehyde. The substance produces sodium hydrogen sulphate addition products as well as a positive iodoform test. It must be a methyl ketone because the chemical isn’t an aldehyde.
A mixture of ethanoic acid and propanoic acid is also produced by the given chemical.
Hence, the given compound is pentan−2−ol.
The reactions can be illustrated by the following equations:

