Solution:
(a) Given rate ![]()
Therefore, order of the reaction ![]()
Dimensions of ![]()
![Rendered by QuickLaTeX.com \[\begin{array}{l} =\frac{\operatorname{mal} L^{-1} \mathrm{~s}^{-1}}{\left(\mathrm{~mol} L^{-1}\right)^{2}} \\ =\frac{\operatorname{mol} L^{-1} \mathrm{~s}^{-1}}{\operatorname{mol}^{-2} L^{-1}} \\ =L \mathrm{~mol}^{-1} \mathrm{~s}^{-1} \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-2cfae784a65dd7a9db018fb1071c6b58_l3.png)
(b) Given rate ![]()
Therefore, order of the reaction ![]()
Dimensions of ![]()
![Rendered by QuickLaTeX.com \[\begin{array}{l} =\frac{m o l}{\left(\operatorname{mol} L^{-1}\right)\left(\operatorname{mol} L^{-1}\right)} \\ =L m o l^{-1} s^{-1} \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-a25e825e1285a9f5915f3a641b81ac7e_l3.png)
(c) Given rate ![]()
Therefore, the order of reaction ![]()
Chemical Kinetics
Dimensions of 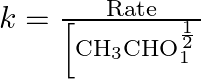
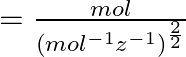
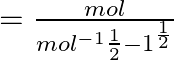
![]()
(d) Given rate ![]()
Therefore, order of the reaction ![]()
Dimension of ![]()
![Rendered by QuickLaTeX.com \[\begin{array}{l} =\frac{\operatorname{mad} L^{-1} s^{-1}}{\operatorname{mal} L^{-1}} \\ =s^{-1} \end{array}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-cf72fada0a7926dca498649064a1f034_l3.png)
