(a) Molality of KI
(b) Molarity of KI
(c) Mole fraction of KI
(a) Molar mass of ![]()
![]() aqueous solution of KI shows that
aqueous solution of KI shows that ![]() of
of ![]() is present in
is present in ![]() of solution.
of solution.
i.e.,
![]() of
of ![]() is present in
is present in ![]() of water
of water ![]() of water
of water
Hence, molality of the solution ![]()
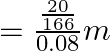
![]()
![]()
(b) The destiny of the solution is given that ![]()
Volume of ![]() solution
solution ![]()
![]()
![]()
![]()
Hence, molarity of the solution 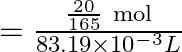
![]()
(c) Moles of ![]()
Moles of water ![]()
Hence, mole ![]()
Fraction of ![]()
![]()
