Answer: (b) F > O > Cl > N During a period, as we move from left to right, the non-metallic characteristics of the elements become more prominent. As a result, F > O > N.
Considering the elements B, C, N, F, and Si, the correct order of their non-metallic character is : a) B > C > Si > N > F b) Si > C > B > N > F c) F > N > C > B > Si d) F > N > C > Si > B
Answer: c) F > N > C > B > Si While moving from left to right in a period, the nonmetallic property of the elements reduces as we move from left to right in the period. As a result, F...
Considering the elements B, Al, Mg, and K, the correct order of their metallic character is : (a) B > Al > Mg > K (b) Al > Mg > B > K (c) Mg > Al > K > B (d) K > Mg > Al > B
Answer: d) K > Mg > Al > B During a period, the metallic nature of the components diminishes as we move from left to right in the period. As a result, Mg > Al. With each group we...
Which one of the following statements is incorrect in relation to ionization enthalpy? (a) Ionization enthalpy increases for each successive electron. (b) The greatest increase in ionization enthalpy is experienced on the removal of an electron from core noble gas configuration. (c) End of valence electrons is marked by a big jump in ionization enthalpy. (d) Removal of an electron from orbitals bearing lower n value is easier than from orbital having higher n value
Answer: (d) is a false statement When comparing orbitals with a lower value of 'n' to orbitals with a higher value of 'n,' it is easier to remove an electron from the lower value of 'n' orbital....
The size of isoelectronic species F–, Ne and Na+ is affected by (a) nuclear charge (Z ) (b) valence principal quantum number (n) (c) electron-electron interaction in the outer orbitals (d) none of the factors because their size is the same.
Answer: (a) Nuclear charge (Z) Because, in the case of isoelectronic species, the atomic size decreases as the number of nuclear charge increases (Z). e.g. the arrangement according to increasing...
“Anything that influences the valence electrons will affect the chemistry of the element”. Which of the factors given below is not affecting the valence shell? (a) Valence Principal quantum number (n) (b) Nuclear charge (Z) (c) Nuclear mass (d) Number of core electrons
Answer: Option c) Because the nucleus is made up of protons and neutrons, the mass of the nucleus has no effect on the valence shell. Protons, or nuclear charges, have an effect on the valence...
Which of the following statements related to the modern periodic table is incorrect? (a) The p-block has 6 columns because a maximum of 6 electrons can occupy all the orbitals in a p-shell. (b) The d-block has 8 columns because a maximum of 8 electrons can occupy all the orbitals in a d-subshell. (c) Each block contains a number of columns equal to the number of electrons that can occupy that subshell. (d) The block indicates the value of an azimuthal quantum number (l) for the last subshell that received electrons in building up the electronic configuration.
Answer: Correcting the incorrect statement is represented by option (b). It explains that "the d-block has 8 columns because a maximum of 8 electrons can occupy all of the orbitals in a d-subshell,"...
In the modern periodic table, the period indicates the value of : (a) atomic number (b) atomic mass (c) principal quantum number (d) azimuthal quantum number.
Answer: (c) The period in the Modern periodic table indicates the value of ‘n’ i.e. a principal quantum number. A periodic table's period reflects the value of a particular element's major quantum...
Predict the formulas of the stable binary compounds that would be formed by the combination of the following pairs of elements. (a) Lithium and oxygen (b) Magnesium and nitrogen (c) Aluminium and iodine (d) Silicon and oxygen (e) Phosphorus and fluorine (f) Element 71 and fluorine
Answer: i) $Li_2O$ is formed when the alkali metal lithium (with one valence electron) and group 16 element oxygen (with two valence electrons) mix. (ii) The alkaline earth metal magnesium (which...
The first 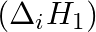 and the second
and the second 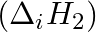 ionization enthalpies (in
ionization enthalpies (in 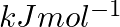 ) and the
) and the 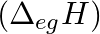 electron gain enthalpy (in
electron gain enthalpy (in 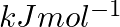 ) of a few elements are given below: Which of the above elements is likely to be : (a) the least reactive element. (b) the most reactive metal. (c) the most reactive non-metal. (d) the least reactive non-metal. (e) the metal which can form a stable binary halide of the formula MX2 (X=halogen). (f) the metal which can form a predominantly stable covalent halide of the formula MX (X=halogen)?
) of a few elements are given below: Which of the above elements is likely to be : (a) the least reactive element. (b) the most reactive metal. (c) the most reactive non-metal. (d) the least reactive non-metal. (e) the metal which can form a stable binary halide of the formula MX2 (X=halogen). (f) the metal which can form a predominantly stable covalent halide of the formula MX (X=halogen)?
Elements (\Delta _{i}H_{1})(ΔiH1) (\Delta _{i}H_{2})(ΔiH2) (\Delta _{eg}H)(ΔegH) 1 520 7300 -60 2 419 3051 -48 3 1681 3374 -328 4 1008 1846 -295 5 2372 5251 +48 6 738 1451 -40 Answer:...
Assign the position of the element having outer electronic configuration (i) 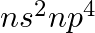 for
for 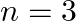 (ii) (n1)
(ii) (n1) 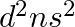 for
for 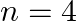 , and (iii)
, and (iii) 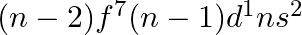 for
for 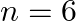 , in the periodic table.
, in the periodic table.
Answer: i) Since n = 6, the element is in period 6. f-block element because the last electron enters f-orbital. They are in the third group. So, 54 + 7 + 2 + 1 Equals 64. So Gadolinium is required....
Write the general outer electronic configuration of s-, p-, d- and f- block elements.
Answer: The general outer electronic configuration of s block elements is $ns ^{(1-2)}$. The general outer electronic configuration of p block elements is $ns ^{2} np ^{(1-6)}$. The general outer...
The increasing order of reactivity among group 1 elements is Li < Na < K < Rb Cl > Br > I. Explain.
Answer: Group 1 elements have only one valence electron. So they tend to expel this electron to form a stable inert gas. The ionization enthalpies of the elements drop in group 1. Expulsion of the...
Use the periodic table to answer the following questions. (a) Identify an element with five electrons in the outer subshell. (b) Identify an element that would tend to lose two electrons. (c) Identify an element that would tend to gain two electrons. (d) Identify the group having metal, non-metal, liquid as well as gas at the room temperature
Answer: (a) The electrical configuration of an element with 5 electrons in the outer subshell is ns2np5. The electrical configuration of the Halogen group is the same. As a result, the elements...
What are the major differences between metals and non-metals?
Answer: Metals lose electrons to produce cations. Metals have low ionization, negative electron, and electronegativities enthalpies. ionic compounds and basic oxides Non-metals readily take...
Would you expect the first ionization enthalpies for two isotopes of the same element to be the same or different? Justify your answer
Answer: The ionization enthalpy of any atom is determined by the number of protons and electrons in the atom. However, the isotopes of any element have the same number of electrons and protons as...
Describe the theory associated with the radius of an atom as it (a) gains an electron (b) loses an electron
Answer: a) An atom loses one electron when it expels one, but the nuclear charge remains the same. In an atom, electron-electron repulsion reduces. So the effective nuclear charge increases. As a...
How would you react to the statement that the electronegativity of N on the Pauling scale is 3.0 in all the nitrogen compounds?
Answer: Electronegativity is a property of any element that is subject to change. Electronegativity varies depending on the substance being studied. The following statement is erroneous: "The...
What is the basic difference between the terms electron gain enthalpy and electronegativity?
Answer: Electron gain enthalpy Electronegativity It is the common tendency of an atom to attract outside electrons It is the general tendency of an atom to attract shared pair of electrons It is the...
Would you expect the second electron gain enthalpy of O as positive, more negative or less negative than the first? Justify your answer
Answer: Oxygen is in p block element group 16. It is the first of 16 members. It is a non-metal found in the earth's crust. When an electron is added to an O atom, it releases energy. O has a...
Which of the following pairs of elements would have a more negative electron gain enthalpy? (i) O or F (ii) F or Cl
Answer: i) O and F are from the same period. As the electron is inserted in the same shell, the atomic size of O-atom is larger than F-atom. It has one less proton than F-atom. So an O-atom nucleus...
The first ionization enthalpy values (in kJ/mol) of group 13 elements are given below. How would you explain this deviation from the general trend?
B Al Ga In Tl 801 577 579 558 589 Answer The ionization enthalpy decreases with group size. B and Al agree. Ga has a higher ionization enthalpy than Al. Tend electrons in the inner shell of...
What are the various factors due to which the ionization enthalpy of the main group elements tends to decrease down a group?
Answer: The factors that cause the ionization enthalpy to drop as we travel down the main group are as follows: 1. “Inner shells increase in number as we proceed down the group.” The nucleus' inner...
How would you explain the fact that the first ionization enthalpy of sodium is lower than that of magnesium but its second ionization enthalpy is higher than that of magnesium?
Answer: Because magnesium has a larger 1st ionization enthalpy than sodium, Magnesium has bigger atoms than sodium. A higher effective nuclear charge than sodium, Magnesium So sodium requires less...
Among the second period elements the actual ionization enthalpies are in the order Li < B < Be < C < O < N < F < Ne. Explain Why {i}Be has higher ΔH than B (ii) O has lower ΔH than N and F?
Answer: i) The electron that can be evacuated from Be(beryllium) atom is 2s, but the electron that can be expelled from boron is 2p. 2s – electron, and nucleus attract more strongly than 2s –...
Energy of an electron in the ground state of the hydrogen atom is 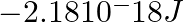 . Calculate the ionization enthalpy of atomic hydrogen in terms of J mol–1.
. Calculate the ionization enthalpy of atomic hydrogen in terms of J mol–1.
Answer: Given that, the electron of hydrogen is having $-2.18 * 10^{-18} J$ in the ground state. Thus, To eject an electron from the ground state in a $H ($ hydrogen $)-$ atom, a certain amount of...
What is the significance of the terms — ‘isolated gaseous atom’ and ‘ground state’ while defining the ionization enthalpy and electron gain enthalpy?
Answer: In the ground state of an isolated gaseous atom, ionisation enthalpy is required to eject an electron. Even though the atoms are far separated in the gaseous state, there are some attraction...
Explain why cation is smaller and anions larger in radii than their parent atoms?
Answer: It possesses less electrons than the parent atom, but the overall nuclear charge remains the same, resulting in greater attraction of electrons to the nucleus. Cations have smaller radii...
Consider the following species : 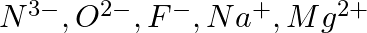 , and
, and 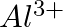 (a) What is common in them? (b) Arrange them in the order of increasing ionic radii.
(a) What is common in them? (b) Arrange them in the order of increasing ionic radii.
Answer: (a) All of the species have the same number of electrons, i.e. 10 electrons. They are, therefore, isoelectronic species. (b) The following is the arrangement of the given ions in ascending...
What do you understand by isoelectronic species? Name a species that will be isoelectronic with each of the following atoms or ions. (i) 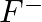 (ii)
(ii) 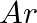 (iii)
(iii) 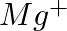 (iv)
(iv) 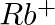
Answer: Isoelectronic species have the same number of electrons as one another. i) The elements $F^-$ and $O_2$ each have ten electrons. As a result, they are considered isoelectronic. (ii) The...
How do atomic radius vary in a period and in a group? How do you explain the variation?
Answer: A period's left to right movement reduces atomic radius. Because external electrons are available in a comparable valence shell, the atomic number increases from left to right, increasing...
What does atomic radius and ionic radius really mean to you?
Answer: The atomic radius is the size of an atom. It measures an atom's size. If an element is a metal, its radius is metallic, and if it is not a metal, its radius is covalent. The metallic radius...
Why do elements in the same group have similar physical and chemical properties?
Answer: The number of valence electrons in any element determines the chemical and physical properties of that element. When elements in the same group of the periodic table have the same number of...
Which element do you think would have been named by (i) Lawrence Berkeley Laboratory (ii) Seaborg’s group?
Answer: (i) Lawrencium (Lr), which has an atomic number of Z=103, and Berkelium (Bk), which has an atomic number of Z=97, are the two elements with the highest atomic numbers. (ii) Seaborgium (Sg),...
What is the atomic number of element keeping in mind both the cases given below; i) Element is in 3rd period of the periodic table. ii) Element is in 17th group of the periodic table.
Answer: The first phase has two elements, while the second period has eight. As a result, element Z = 11 starts the third phase. The third period currently has eight elements. As a result, the 18th...
In terms of period and group where would you locate the element with Z =114?
Answer: The seventh period of the periodic table contains elements with atomic numbers Z = 87–114. The element with Z = 114 is thus available in the seventh period. In the seventh period, the first...
On the basis of quantum numbers, justify that the sixth period of the periodic table should have 32 elements.
Answer: For the outermost shells of a periodic table, a period shows the value of the main quantum number (n). Each period begins with the primary quantum number (n). And n for the 6th period is 6....
What is the basic difference in approach between the Mendeleev’s Periodic Law and the Modern Periodic Law?
Answer: Mendeleev’s Approach for periodic law Modern approach for the periodic law Periodic functions of the atomic mass of the corresponding elements determine the chemical and physical properties...
Which important property did Mendeleev use to classify the elements in his periodic table and did he stick to that?
Answer: Mendeleev arranged the elements in his periodic table by atomic weight. Mendeleev classified the elements into groups and periods based on atomic weight. Mendeleev grouped elements with...
What is the basic theme of organization in the periodic table?
Answer: It divides items into periods and groups based on their qualities. This method simplifies and organizes the study of elements and their compounds. Elements with similar characteristics are...
Among halogens, the correct order of the amount of energy released in electron gain (electron gain enthalpy) is:
(i) F > Cl > Br > I
(ii) F < Cl < Br < I
(iii) F < Cl > Br > I
(iv) F < Cl < Br < I
Option (iii) is the answer. The amount of energy released when an electron is added to an isolated gaseous atom is known as electron gain enthalpy. The electron gain enthalpy of F is less...
The electronic configuration of gadolinium (Atomic number 64) is
![Rendered by QuickLaTeX.com \[\left( \mathbf{i} \right)\text{ }\left[ \mathbf{Xe} \right]\text{ }\mathbf{4f}{{~}^{\mathbf{3}}}~\mathbf{5}{{\mathbf{d}}^{\mathbf{5}}}~\mathbf{6}{{\mathbf{s}}^{\mathbf{2}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-0053fdb46edb1b5c5c8a11e78990291a_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{ii} \right)\text{ }\left[ \mathbf{Xe} \right]\text{ }\mathbf{4}{{\mathbf{f}}^{\mathbf{7}}}~\mathbf{5d2}\text{ }\mathbf{6}{{\mathbf{s}}^{\mathbf{1}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-0eea84eed0ff28f2a34c3dd7fdeaa933_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{iii} \right)\text{ }\left[ \mathbf{Xe} \right]\text{ }\mathbf{4}{{\mathbf{f}}^{\mathbf{7}}}~\mathbf{5}{{\mathbf{d}}^{\mathbf{1}}}~\mathbf{6}{{\mathbf{s}}^{\mathbf{2}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-d9130ba366bc1be1b7d92e3b05d87326_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{iv} \right)\text{ }\left[ \mathbf{Xe} \right]\text{ }\mathbf{4}{{\mathbf{f}}^{\mathbf{8}}}~\mathbf{5}{{\mathbf{d}}^{\mathbf{6}}}~\mathbf{6}{{\mathbf{s}}^{\mathbf{2}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-c4766769bfc3513e1f8e729a3f9c95cd_l3.png)
Option (iii) is the answer. The electronic configuration of gandolium is [Xe] 4f7 5d1 6s2
