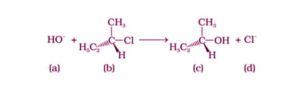
Option (i) and (iv) are the answers.
Because it is a tertiary halide, it undergoes the SN1 process, resulting in the formation of a carbocation as an intermediate.

Option (i) and (iv) are the answers.
Because it is a tertiary halide, it undergoes the SN1 process, resulting in the formation of a carbocation as an intermediate.