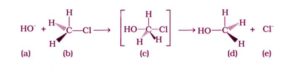
Option (i) and (iii) are the answers.
Nucleophiles are HO and CF. The C atom is sp2 hybridised in (iii) due to the simultaneous creation of the C – OH bond and the breakdown of the C – Cl link. As a result, in the transition state, the C atom is fully linked to only three H atoms.
