Solution: Radius of each circular plate is given as $0.06m$ Capacitance of a parallel plate capacitor is given as $\mathrm{C}=100 \mathrm{pF}=100 \times 10^{-12} \mathrm{~F}$ Supply voltage is given...
A parallel plate capacitor made of circular plates each of radius
What are anti-oxidants? Why are they added to fat and oil containing foods?
Antioxidants are compounds that are added to fat and oil-containing foods to help slow down or prevent rancidity. Antioxidants can be nutrients as well as enzymes that are proteins that aid in...
A parallel plate capacitor made of circular plates each of radius 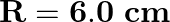 has a capacitance
has a capacitance 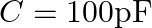 . The capacitor is connected to a
. The capacitor is connected to a 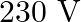 ac supply with an (angular) frequency of
ac supply with an (angular) frequency of 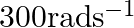 .
.
(a) What is the rms value of the conduction current?
(b) Is the conduction current equal to the displacement current?
Solution: Radius of each circular plate is given as $0.06m$ Capacitance of a parallel plate capacitor is given as $\mathrm{C}=100 \mathrm{pF}=100 \times 10^{-12} \mathrm{~F}$ Supply voltage is given...
State important uses of decomposition reactions.
Important uses of decomposition reactions:- (i) In photography, light is utilized to decompose silver chloride into silver and chlorine. This is an important use of decomposition reaction. (ii) This...
The Figure shows a capacitor made of two circular plates each of radius 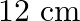 and separated by
and separated by 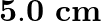 . The capacitor is being charged by an external source (not shown in the figure). The charging current is constant and equal to 0.15A. Is Kirchhoff’s first rule (junction rule) valid at each plate of the capacitor? Explain.
. The capacitor is being charged by an external source (not shown in the figure). The charging current is constant and equal to 0.15A. Is Kirchhoff’s first rule (junction rule) valid at each plate of the capacitor? Explain.
Solution: Yes Kirchhoff's first rule holds true for each capacitor plate if we use the total of conduction and displacement to calculate current.
What is the general name of the chemicals which are added to fat and oil containing foods to prevent the development of rancidity?
Antioxidants are compounds that are added to fat and oil-containing foods to help slow or prevent rancidity. Ascorbic acid and tocopherols are natural antioxidants, but butylated hydroxyanisole...
The Figure shows a capacitor made of two circular plates each of radius 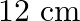 and separated by
and separated by 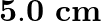 . The capacitor is being charged by an external source (not shown in the figure). The charging current is constant and equal to 0.15A.
. The capacitor is being charged by an external source (not shown in the figure). The charging current is constant and equal to 0.15A.
(a) Calculate the capacitance and the rate of change of the potential difference between the plates.
(b) Obtain the displacement current across the plates.
The radius of each circular plate $(r)$ is given as $0.12 \mathrm{~m}$ The distance between the plates $(d)$ is given as $0.05 \mathrm{~m}$ The charging current (I) is given as $0.15 \mathrm{~A}$...
Which term is used to indicate the development of unpleasant smell and taste in fat and oil-containing foods due to aerial oxidation (when they are kept exposed for a considerable time)?
The term "rancidity" is used to describe the situation caused by the aerial oxidation of unsaturated fat found in food and other items. When fats and oils are exposed to air, light, or moisture,...
Write the balanced chemical equation for the following reaction: Zinc + Silver nitrate Zinc nitrate + Silver
The balanced chemical equation for the reaction of zinc with silver nitrate to give zinc nitrate and silver. Zn + 2AgNO3 → Zn(NO3)2 + 2Ag
Why does the colour of copper sulphate solution change when an iron nail is kept immersed in it?
It is a displacement reaction. Because an iron nail dropped in the solution is more reactive than copper sulphate and displaces the molecules, the copper sulphate solution changes colour. As the...
Name the various types of chemical reactions.
Chemical reactions come in a variety of forms. The following are the names: (i) Combination reactions. (ii) Decomposition reaction. (iii) Displacement reaction. (iv) Double displacement reaction....
What type of reaction is represented by the digestion of food in our body?
Our digestive system represents the decomposition reaction. It is nothing more than a chemical reaction in which the food we are eating is further broken down into digestible nutrients such as fats,...
Gas A, which is the major cause of global warming, combines with hydrogen oxide B in nature in the presence of an environmental factor C and a green material D to form a six carbon organic compound E and a gas F. The gas F is necessary for breathing.(a) What is gas A?(b) What is the common name B?(c) What do you think could be C?(d) What is material D? Where is it found? (e) Name the organic compound E.(f) What is gas F? Name the natural process during which it is released.
(a) Gas A is Carbon dioxide, CO2 (b) B is Water, H2O (c) Sunlight is the environmental factor (d) Material D is Chlorophyll; it is found in green leaves of plants which provide pigmentation (e)...
The white solid compound A decomposes quite rapidly on heating in the presence of a black substance X to form a solid compound B and a gas C. When an aqueous solution of compound B is reacted with silver nitrate solution, then a white precipitate of silver chloride is obtained alongwith potassium nitrate solution. Gas C does not burn itself but helps burn things. (a) What is compound A? (b) What is compound B? (c) What is gas C? (d) What do you think is the black substance X? What is its function? (e) What is the general name of substance like X?
(a) Compound A is Potassium chlorate, KClO3 (b) Compound B is Potassium chloride, KCl (c) Gas C is Oxygen, O2 (d) X is Manganese dioxide, MnO2; Manganese dioxide is used as a catalyst in order to...
When a mixture of gases X and Y is compressed to 300 atm pressure and then passed over a catalyst consisting of a mixture of zinc oxide and chromium oxide (heated to a temperature of 300oC), then an organic compound Z having the molecular formula CH4O is formed. X is a highly poisonous gas which is formed in appreciable amounts when a fuel burns in a limited supply of air; Y is a gas which can be made by the action of a dilute acid on an active metal; and Z is a liquid organic compound which can react with sodium metal to produce hydrogen gas. (a) What are X, Y and Z? (b) Write a balance chemical equation of the reaction which takes place when X and Y combine to form Z. Indicate the conditions under which the reaction occurs.
(a) X= Carbon monoxide gas (CO); Y= Hydrogen gas (H2); Z = Methanol (CH3OH or CH4O) (b)CO(g) + 2H2(g) → CH3OH (l) Carbon monoxide when reacts with hydrogen gas under a pressure of 300atmosphere and...
The metal M reacts vigorously with water to form a solution S and a gas G. The solution S turns red litmus to blue whereas gas G, which is lighter than air, burns with a pop sound. Metal M has a low melting point and is used as a coolant in nuclear reactors. (a) What is metal M? (b) What is solution S? Is it acidic or alkaline? (c) What is gas G? (d) Write a balanced chemical equation for the reaction which takes place when metal M reacts with water. (e) Is this reaction exothermic or endothermic?
(a) Sodium is the metal ( Na ) (b) Solution S is Sodium hydroxide solution (NaOH solution),It is alkaline. (c) Hydrogen, H2is the gas evolved (d) 2Na + 2H2O → 2NaOH + H2 (e) Reaction is...
A metal X forms a salt XSO4. The salt XSO4 forms a clear solution in water which reacts with sodium hydroxide solution to form a blue precipitate Y. Metal X is used in making electric wires and alloys like brass. (a) What do you think metal X could be? (b) Write the name, formula, and colour of salt XSO4. (c) What is the blue precipitate Y? (d) Write a chemical equation of the reaction which takes place. When salt XSO4 reacts with sodium hydroxide solution. Give the state symbols of all the reactants and products which occur in the above equation.
(a) Metal could be Copper, Cu. (b) Copper sulphate, CuSO4, Blue colour. (c) The blue precipitate Y is Copper hydroxide, Cu(OH)2 (d) CuSO4 (aq) + 2NaOH (aq) → Cu(OH)2 (s) +...
A silvery-white metal X taken in the form of ribbon, when ignited, burns in air with a dazzling white flame to form a white powder Y. When water is added to powder Y, it dissolves partially to form another substance Z. (a) What could metal X be? (b) What is powder Y? (c) With which substance metal X combines to form powder Y? (d) What is substance Z? Name one domestic use of substance Z. (e) Write a balanced chemical equation of the reaction which takes place when metal X burns in air to form powder Y.
(a) Magnesium, Mg because magnesium undergoes oxidation in the presence of oxygen. (b) Magnesium oxide, MgO (c) Oxygen (of air), O2 (d) Magnesium hydroxide, Mg(OH)2; Used as an antacid to relieve...
A solid substance P which is very hard is used in the construction of many buildings, especially flooring. When substance P is heated strongly, it decomposes to form another solid Q and a gas R is given out. Solid Q reacts with water with the release of a lot of heat to form a substance S. When gas R is passed into a clear solution of substance S, then a white precipitate of substance T is formed. The substance T has the same chemical composition as starting substance P. (a) What is substance P? Write its common name as well as a chemical formula. (b) What is substance Q? (c) What is gas R? (d) What is substance S? what is its clear solution known as? (e) What is substance T? Name any two natural forms in which substance T occurs in nature.
(a) Calcium carbonate (limestone), CaCO3 (b) Calcium oxide, CaO (c) Carbon dioxide, CO2 (d) Calcium hydroxide, Ca(OH)2; Lime water. (e) Calcium carbonate; Limestone and Marble.
When metal X is treated with a dilute acid Y, then a gas Z is evolved which burns readily by making a little explosion. (a) Name any two metals which can behave like metal X. (b) Name any two acids which can behave like acid Y. (c) Name the gas Z. (d) Is the gas Z lighter than or heavier than air? (e) Is the reaction between metal X and dilute acid Y exothermic or endothermic? (f) By taking a specific example of metal X and dilute acid Y, Write a balanced chemical equation for the reaction which takes place. Also, indicate physical states of all the reactants and products.
(a) Zinc and Iron. (b) Dilute hydrochloric acid and dilute sulphuric acid. (c) Hydrogen. (d) Lighter than air. (e) Exothermic. (f) Suppose metal X is zinc (Zn) and acid Y is dilute hydrochloric acid...
Explain the following.
a. Why is the tungsten used almost exclusively for filament of electric lamps?
b. Why are the conductors of electric heating devices, such as bread-toasters and electric irons, made of an alloy rather than a pure metal?
c. Why is the series arrangement not used for domestic circuits?
d. How does the resistance of a wire vary with its area of cross-section?
e. Why copper and aluminium wires are usually employed for electricity transmission?
a. Tungsten has a high resistance and melting point. It doesn't burn easily when heated because of its characteristic. Electric bulbs generate a lot of heat. As a result, tungsten is a popular metal...
An electric heater of resistance 8 Ω draws 15 A from the service mains 2 hours. Calculate the rate at which heat is developed in the heater.
The following formula can be used to compute the rate at which heat develops in the heater. I2 R = P We obtain by substituting the numbers in the equation. P = (15A) 2 × 8 Ω = 1800 J/s Heat is...
Which uses more energy, a 250 W TV set in 1 hr, or a 1200 W toaster in 10 minutes?
The equation gives the amount of energy consumed by electrical appliances. H = Pt, where P is the appliance's power and t is the time. The energy consumed by a TV with a power rating of 250 W may be...
Two lamps, one rated 100 W at 220 V, and the other 60 W at 220 V, are connected in parallel to electric mains supply. What current is drawn from the line if the supply voltage is 220 V?
The voltage across both bulbs will be the same because they are linked in parallel. The current drawn by a 100 W bulb may be calculated as follows: P/V = I We obtain by substituting the numbers in...
Compare the power used in the 2 Ω resistor in each of the following circuits: (i) a 6 V battery in series with 1 Ω and 2 Ω resistors, and (ii) a 4 V battery in parallel with 12 Ω and 2 Ω resistors.
(i) Because the resistors 1 and 2 are linked in series and the potential difference is 6 V, their equivalent resistance is 1 + 2 = 3. Using Ohm's law, the current in the circuit may be computed. As...
A hot plate of an electric oven connected to a 220 V line has two resistance coils A and B, each of 24 Ω resistance, which may be used separately, in series, or in parallel. What are the currents in the three cases?
Case1 :When coils are utilised individually in case I We can calculate the current going through each coil using Ohm's law : When each resistor is utilised independently, 9.166 A of current passes...
The number of carbon atoms joined in a spherical molecule of buckminsterfullerene is:
(a) Fifty (b) Sixty (c) Seventy (d) Ninety Solution: Option (b) is the answer. Buckminsterfullerene has the formula C60 and is a kind of fullerene. It is made up of twenty hexagons and twelve...
The number of isomers formed by the hydrocarbon with molecular formula C5H12 is:
(a) 2 (b) 5 (c) 3 (d) 4 Solution: Option (c) is the answer.
The pencil leads are made of mainly:
(a) Lithium (b) Charcoal (c) Lead (d) Graphite Solution: Option (d) is the answer.
Which of the following cannot exhibit isomerism?
(a) C4H10 (b) C5H12 (c) C3H8 (d) C6H14 Solution: Option (c) is the answer. Because there must be greater than or equal to 4 carbon atoms for chain isomerism to occur, propane does not display chain...
The hydrocarbon which has alternate single and double bonds arranged in the form of a ring is:
(a) Cyclobutane (b) Benzene (c) Butene (d) Hexene Solution: Option (b) is the answer. Benzene is a hydrocarbon that has alternate single and double bonds arranged in the form of a ring. Alternating...
A hydrocarbon having one double bond has 100 carbon atoms in its molecule. The number of hydrogen atoms in its molecule will be:
(a) 200 (b) 198 (c) 202 (d) 196 Solution: Option (a) is the answer. Alkenes are made up of double bonds and have the formula CnH2n. The number of carbon atoms in its molecule is denoted by the...
A saturated hydrocarbon has fifty hydrogen atoms in its molecule. The number of carbon atoms in its molecule will be:
(a) Twenty-five (b) Twenty four (c) Twenty-six (d) Twenty seven Solution: Option (b) is the answer. Saturated hydrocarbons with the general formula CnH2n+2 are known as alkanes. The number of...
The pair of elements that exhibits the property of catenation is :
(a) Sodium and silicon (b) Chlorine and carbon (c) Carbon and sodium (d) Silicon and carbon Solution: Option (d) is the answer. Silicon and Carbon both can bond with 4 other atoms to form long-chain...
The number of carbon atoms in the organic compound named 2,2-dimethylpropane is:
(a) Two (b) Five (c) Three (d) Four Solution: Option (b) is the answer. Neopentane, commonly known as 2,2-dimethylpropane, is a five-carbon double-branched-chain alkane. At room temperature and...
What is the function of an earth wire? Why is it necessary to earth metallic appliances?
Earth wire connects the metallic body of electric equipment to the earth. Any electric wire leakage is transported to the ground via the earth wire. This prevents electric shocks to the user of the...
One of the following is not an allotrope of carbon. This is:
(a) Diamond (b) Graphite (c) Cumene (d) Buckminsterfullerene Solution: Option (c) is the answer. Cumene (isopropylbenzene) is an aliphatic-substituted aromatic hydrocarbon. Crude oil and refined...
When does an electric short circuit occur?
An electric short circuit can occur When too many appliances are connected to a single socket, the circuit's resistance decreases. It causes the current flowing through it to increase dramatically....
When water is added gradually to a white solid X. A hissing sound and a lot of heat is produced forming a product Y. A suspension of Y in water is applied to the walls of a house during whitewashing. A clear solution of Y is also used for testing carbon dioxide gas in the laboratory. (a) What could be solid X? Write its chemical formula. (b) What could be product Y? Write its chemical formula. (c) What is the common name of the solution of Y which is used for testing carbon dioxide gas? (d) Write a chemical equation of the reaction which takes place on adding water to solid X. (e) Which characteristics of chemical reactions are illustrated by this example?
Answer: (a) Calcium oxide, CaO. (b) Calcium hydroxide, Ca(OH)2 (c) Lime water. (d) CaO + H2O → Ca(OH)2 (e) Change in temperature.
When the solution of substance X is added to a solution of potassium iodide, then a yellow solid separates out from the solution. (a) What do you think substance X is likely to be? (b) Name the substance of which the yellow solid consists. (c) Which characteristic of chemical reactions is illustrated by this example? (d) Write a balanced chemical equation for the reaction which takes place. Mention the physical states of all the reactants and products involved in the chemical equation.
Answer: (a) Lead Nitrate (b) Lead Iodide (c) Formation of precipitate (d)Pb(NO3)2 (aq) + 2KI (aq) → PbI2 (s) + 2KNO3 (aq)
The organic compound prepared by Wohler from an inorganic compound called ammonium cyanate was;
(a) Glucose (b) Urea (c) Uric acid (d) Vinegar Solution: Option (b) is the answer.
The chemical equations are balanced to satisfy one of the following laws in chemical reactions. This law is known as (a) Law of conservation of momentum (b) Law of conservation of mass (c) Law of conservation of motion (d) Law of conservation of magnetism
Answer: Option (b) is the correct response. When the mass of atoms of different elements in the reactants side equals the mass of atoms in the products side, the equations are balanced.
A diamond-toothed saw is usually for cutting;
(a) Steel girders (b) Logs of woods (c) Marble slabs (d) Asbestos sheets Solution: Option (c) is the answer. Diamond is hard. Hence suitable for cutting marble slabs.
Explain the underlying principle and working of an electric generator by drawing a labelled diagram. What is the function of brushes?
The mechanical energy is converted into electrical energy by the electric generator. Electromagnetic induction is the basis of the electric generator's operation. It works by rotating a coil in a...
One of the following is an exothermic reaction. This is: (a) Electrolysis of water (b) Conversion of limestone into quicklime (c) Process of respiration (d) Process of photosynthesis
Answer: Option (c) is the correct response. As heat energy is emitted during respiration, it is an exothermic reaction that keeps our body temperature stable.
Which of the following is not an endothermic reaction? (a) CaCO3→ CaO + CO2 (b) 2H2O → 2H2 + O2 (c) 6CO2 + 6H2O → C6H12O6 + 6O2 (d) C6H12O6 + 6O2→ 6CO2 + 6H2O
Answer: Option (d) is the correct response. This is responsible for the exothermic respiration reaction, in which glucose burns in oxygen to produce heat energy.
An alkyne has seventy-five carbon atoms in its molecule. The number of the hydrogen atoms in its molecule will be :
(a) 150 (b) 148 (c) 152 (d) 146 Solution: Option (b) is the answer. The number of hydrogen atoms in an alkyne molecule with 75 carbon atoms is 148. Alkynes have the generic formula CnH2n-2, where n...
State the rule to determine the direction of a current induced in a coil due to its rotation in a magnetic field.
Fleming’s right-hand rule
State the rule to determine the direction of a (i) magnetic field produced around a straight conductor-carrying current, (ii) force experienced by a current-carrying straight conductor placed in a magnetic field which is perpendicular to it.
(i) Maxwell's right-hand thumb rule (ii) Fleming's left hand rule
One of the following is an endothermic reaction. This is: (a) Combination of carbon and oxygen to form carbon monoxide (b) Combination of nitrogen and oxygen to form nitrogen monoxide (c) Combination of glucose and oxygen to form carbon dioxide and water(d) Combination of zinc and hydrochloric acid to form zinc chloride and hydrogen
Answer: Option (b) is the correct response. Nitrogen monoxide is formed when nitrogen and oxygen are heated to extremely high temperatures in an endothermic reaction.
An unsaturated hydrocarbon having a triple bond has 50 hydrogen atoms in the molecule. The number of carbon atoms in its molecule will be:
(a) 24 (b) 25 (c) 26 (d) 28 Solution: Option (c) is the answer. CnH2n-2 is the general formula for unsaturated hydrocarbons containing a triple bond. The number of atoms in one molecule is denoted...
The chemical reaction between quicklime and water is characterised by: (a) Evolution of hydrogen gas (b) Formation of a slaked lime precipitate (c) Change in temperature of mixture (d) Change in colour of the product
Answer: Option (c) is the correct response. The reaction between quicklime and water produces a greater amount of energy, making it an exothermic reaction.
Two circular coils A and B are placed closed to each other. If the current in the coil A is changed, will some current be induced in the coil B? Give reason.
The magnetic field associated with coil A changes as the current in it changes. As a result, the magnetic field surrounding coil B shifts. The magnetic field of coil B changes, causing current to...
The hydrocarbon 2-methyl butane is an isomer of :
(a) n-pentane (b) n-butane (c) Propane (d) Iso-butane Solution: Option (a) is the answer.
The chemical reaction between the two substances is characterised by a change in colour from orange to green. These two substances are most likely to be: (a) Potassium dichromate solution and sulphur dioxide (b) Potassium permanganate solution and sulphur dioxide (c) Potassium permanganate solution and lemon juice (d) Potassium dichromate solution and carbon dioxide
Answer: Option (a) is the correct response. The reaction of potassium dichromate with sulphur dioxide is characterised by a colour shift. By passing sulphur dioxide through acidified potassium...
A coil of insulated copper wire is connected to a galvanometer. What will happen if a bar magnet is held stationary inside the coil?
There will be no current induced while the bar magnet is held stationary inside the coil. Therefore there will be no deflection in the galvanometer.
A coil of insulated copper wire is connected to a galvanometer. What will happen if a bar magnet is (i) pushed into the coil, (ii) withdrawn from inside the coil
(i) When a bar magnet is placed into the coil, The current is induced in the coil for a brief period of time. This causes the galvanometer to deflect in a specific direction. (ii) When the bar...
An acid that can decolorise purple coloured potassium permanganate solution is : (a) Sulphuric acid (b) Citric acid (d) Carbonic acid (d) Hydrochloric acid
Answer: Option (b) is the correct response. When purple-colored potassium permanganate reacts with citric acid, the colour changes from purple to colourless.
A cyclic hydrocarbon having carbon-carbon single bonds as well as carbon-carbon double bonds in the molecule is:
(a) C6H12 (b) C6H14 (c) C6H6 (d) C6H10 Solution: Option (c) is the answer.
You are given the solution of lead nitrate. In order to obtain a yellow precipitate, you should mix it with a solution of (a) Potassium chloride (b) Potassium nitride(c) Potassium sulphide (d) Potassium iodide
Answer: Option (d) is the correct response. When lead nitrate reacts with potassium iodide, a yellow precipitate of lead iodide is formed.
The property of self-combination of the atoms of the same element to form long chains is known as:
(a) Protonation (b) Carbonation (c) Coronation (d) Catenation Solution: Option (d) is the answer. Catenation is the bonding of atoms of the same element into a series, known as a chain, in...
Name some devices in which electric motors are used.
Some devices which uses electric motors are: MixersWater pumpsElectric fansWashing machines
Which one of the following does not involve a chemical reaction? (a) Digestion of food in our body (b) Process of respiration (c) Burning of candle wax when heated (d) Melting of candle wax on heating
Answer: Option (d) is the correct response. Wax changes its physical state but not its characteristics when heated, therefore there is no chemical change but there is a physical change.
The number of covalent bonds in pentane (molecular formula C5H12) is:
(a) 5 (b) 12 (c) 17 (d) 16 Solution: Option (d) is the answer. In pentane, each bond pair is a covalent bond.There are 16 single bonds in all.As a result, the correct answer is 16.
One of the following does not happen during a chemical reaction This is : (a) Breaking of old chemical bonds and formation of new chemical bonds (b) Formation of new substances with entirely different properties (c) Atoms of one element change into those of another element to form new products(d) A rearrangement of atoms takes place to form new products
Answer: Option (c) is the correct response. Atoms of one element do not convert into another to generate products; however, bonds between these atoms break and products are formed, and atoms are...
Draw a labelled diagram of an electric motor. Explain its principle and working. What is the function of a split ring in an electric motor?
A device that transforms electrical energy into mechanical energy is known as an electric motor. It operates on the principle of the current magnetic effect. A simple electric motor is seen in the...
Answer the following questions: (a) What do you understand by exothermic and endothermic reactions? (b) Give one example of an exothermic reaction and one of an endothermic reaction. (c) Which of the following are endothermic reactions and which are exothermic reactions? (i) Burning of natural gas (ii) Photosynthesis (iii) Electrolysis of water (iv) Respiration (v) Decomposition of calcium carbonate
Answer: (a)An exothermic reaction occurs when energy is discharged from the system into the environment. An endothermic reaction occurs when energy is taken from the environment in the form of heat....
Imagine that you are sitting in a chamber with your back to one wall. An electron beam, moving horizontally from back wall towards the front wall, is deflected by a strong magnetic field to your right side. What is the direction of magnetic field?
The Fleming's Left hand rule can be used to determine the direction of the magnetic field. The magnetic field will be perpendicular to the current direction and the direction of deflection, i.e.,...
Out of the following pairs of compounds, the unsaturated compounds are:
(a) C2H6 and C4H6 (b) C6H12 and C5H12 (c) C4H6 and C6H12 (d) C2H5 and C4H10 Solution: Option (c) is the answer. Unsaturated hydrocarbons are those that belong to the alkene or...
When is the force experienced by a current–carrying conductor placed in a magnetic field largest?
The force experienced is greatest when the direction of the current is perpendicular to the direction of the magnetic field.
(a) State the various characteristics of chemical reactions. (b) State one characteristic of each of the chemical reactions which take place when: (i) Dilute hydrochloric acid is added to sodium carbonate (ii) Lemon juice is added gradually to potassium permanganate solution (iii) Dilute sulphuric acid is added to barium chloride solution (iv) Quicklime is treated with water (v) Wax is burned in the form of a candle
Answer: (a) A chemical reaction is a process that occurs when reactants react to generate new material. It is defined by the evolution of a gas, color change, precipitate formation, temperature...
How does a solenoid behave like a magnet? Can you determine the north and south poles of a current–carrying solenoid with the help of a bar magnet? Explain.
A solenoid is a large coil of insulated copper wire looped in a circular pattern. When current is passed through the solenoid, the magnetic field produced around it is comparable to that produced...
(a) What is meant by a chemical reaction? Explain with the help of an example. (b) Give one example each of a chemical reaction characterized by: (i) Evolution of a gas (ii) Change in colour (iii) Formation of a precipitate (iv) Change in temperature (v) Change in the state.
Answer: (a) By undergoing particular reactions and rearrangements, a chemical reaction produces new compounds. On the left, there will be reactants, and on the right, there will be products. Methane...
Buckminsterfullerene is an allotropic form of the element:
(a) Phosphorus (b) Fluorine (c) Carbon (d) Sulphur Solution: Option (c) is the answer. Buckminsterfullerene has the formula C60 and is a kind of fullerene. It is made up of twenty hexagons and...
(e) How many isomers of the following hydrocarbons are possible?
(i) C3H8 (ii) C4H10 (iii) C5H12 (iv) C6H14 Solution: (e) (i) No isomers (ii) Two (iii) Three (iv) Five
List two methods of producing magnetic fields.
Two methods for producing magnetic fields are: We can create a magnetic field using a permanent magnet, which may be visualised by distributing iron filings on white paper and placing a magnet...
State whether the following statements are true or false.
a. The field at the center of a long circular coil carrying current will be parallel straight lines.
b. A wire with a green insulation is usually the live wire of an electric supply.
a. True A solenoid is a long circular coil. Inside a solenoid, the magnetic field lines are parallel straight lines. b. False The earth wire has green insulation while the live wires have red...
(c) Give the names and structural formulae of one saturated cyclic hydrocarbon and one unsaturated cyclic hydrocarbon.
(d) Give one example of a hydrocarbon, other than pentane, having more than three isomers. Solution: (c) Saturated cyclic hydrocarbon: Cyclohexane C6H12 Unsaturated Cyclic hydrocarbon: Benzene C6H6...
(a) What are hydrocarbons? Explain with examples.
(b) Explain the meaning of saturated and unsaturated hydrocarbons with two examples each. Solution: (a) A hydrocarbon is a compound composed entirely of hydrogen and carbon. Methane (CH4), ethane...
When potassium nitrate is heated, it decomposes into potassium nitrite and oxygen. Write a balanced equation for this reaction and add the state symbols of the reactants and products.
Answer: The balanced chemical equation for heating of potassium nitrate to decompose into potassium nitrite and oxygen is as follows: 2KNO3 (s)→ 2KNO2 (s) + O2 (g)
State whether the following statements are true or false.
a. An electric motor converts mechanical energy into electrical energy.
b. An electric generator works on the principle of electromagnetic induction.
a. False Electrical energy is converted into mechanical energy by an electric motor. b. True A device that creates electricity by rotating a coil in a magnetic field is known as an electric...
Translate the following statement into a chemical equation and then balance it: ‘Barium chloride solution reacts with aluminium sulphate solution to form a precipitate of barium sulphate and aluminium chloride solution’.
Answer: The balanced chemical equation for the reaction of Barium chloride with Aliminium sulphate solution to form a precipitate of barium sulphate and aluminium chloride solution is as follows:...
At the time of short circuit, the current in the circuit
a. reduces substantially.
b. does not change.
c. increases heavily.
d. vary continuously.
c. increases heavily When two naked wires in a circuit make contact, the amount of current flowing in the circuit abruptly increases, resulting in a short circuit.
The essential difference between an AC generator and a DC generator is that
a. AC generator has an electromagnet while a DC generator has permanent magnet.
b. DC generator will generate a higher voltage.
c. AC generator will generate a higher voltage.
d. AC generator has slip rings while the DC generator has a commutator.
d. AC generator has slip rings while the DC generator has a commutator. The slip rings are two rings on AC generators, while the commutator is two half rings on DC generators. This is the primary...
(c) Write the IUPAC name of the compound having the formula n-C4H10.
(d) Write the IUPAC names for the following: Solution: (c) Butane (d) (i) 2-methylpropane (ii) 2-methylbutane (iii) Propene (iv) Propyne
The device used for producing electric current is called a
a. generator
b. galvanometer
c. ammeter
d. motor
a. generator The generator is the device that produces electric current. The generator is a device that converts mechanical energy into electrical energy.
(a) Explain the term ‘isomers’. Give one example of isomers.
(b) Write (i) structural formula, and (ii) electron-dot structure, of anyone isomer of n-heptane. Solution: (a) Isomers are organic molecules that have the same chemical formula but distinct...
Write a balanced chemical equation for the process of photosynthesis giving the physical states of all the substances involved and the conditions of the reaction.
The balanced chemical equation for the process of photosynthesis which produces carbohydrates and water is as follows: 6CO2 (g) + 6H2O (l) → C6H12O6 (aq.) + 6O2 (g) Thus reaction...
The phenomenon of electromagnetic induction is
a. the process of charging a body.
b. the process of generating magnetic field due to a current passing through a coil.
c. producing induced current in a coil due to relative motion between a magnet and the coil.
d. the process of rotating a coil of an electric motor.
c. producing induced current in a coil due to relative motion between a magnet and the coil. The process of inducing electricity in a coil due to the relative motion of the coil and the magnet is...
Ammonia reacts with oxygen to form nitrogen and water. Write a balanced chemical equation for this reaction. Add the state symbols for all the reactants and products.
Answer: The balanced chemical equation for the reaction of ammonia with oxygen producing nitrogen and water: 4NH3 (g) + 3O2 (g) → 2N2 (g) + 6H2O (l)
Which of the following correctly describes the magnetic field near a long straight wire?
a. The field consists of straight lines perpendicular to the wire.
b. The field consists of straight lines parallel to the wire.
c. The field consists of radial lines originating from the wire.
d. The field consists of concentric circles centered on the wire.
d. The field consists of concentric circles centered on the wire. Concentric circles form the magnetic field around a long straight wire. Their centres are on the wire.
(a) Substitute formulae for names and balance the following equations: Calcium carbonate reacts with hydrochloric acid to produce calcium chloride, water, and carbon dioxide gas. (b) Write a balanced chemical equation with state symbols for the following reaction: Sodium hydroxide solution reacts with a hydrochloric acid solution to produce sodium chloride solution and water.
Answer: (a) Balanced chemical equation for the reaction of calcium carbonate with hydrochloric acid: CaCO3 + 2HCl → CaCl2 + H2O + CO2 (b) Balanced chemical equation for the...
What precaution should be taken to avoid the overloading of domestic electric circuits?
Precautions to be taken to avoid overloading of domestic electric circuits are: Avoid connecting too many devices to a single socket.Avoid using too many appliances simultaneously.Faulty appliances...
(a) Potassium chlorate (KClO3) on heating forms potassium chloride and oxygen. Write a balanced equation for this reaction and indicate the evolution of gas. (b) Rewrite the following information in the form of a balanced chemical equation: Magnesium burns in carbon dioxide to form magnesium oxide and carbon.
Answer: (a) Balanced chemical equation for heating of potassium chlorate: 2KClO3 (s) → 2KCl (s) + 3O2 (g) (b) Balanced chemical equation for burning of magnesium in carbon...
An electric oven of 2 kW power rating is operated in a domestic electric circuit (220 V) that has a current rating of 5 A. What result do you expect? Explain.
The current drawn by the electric oven can be calculated using the formula P = V × I I = P/V On putting the values, we get I = 2000 W/220 V = 9.09 A The current drawn by the...
Name two safety measures commonly used in electric circuits and appliances.
The two safety measures are: Fuse: A fuse should be connected to each circuit because it prevents excessive current from flowing through the circuit. When the current in the circuit exceeds the fuse...
Carbon monoxide reacts with hydrogen under certain conditions to form methanol. Write a balanced chemical equation for this reaction indicating the physical states of reactants and products as well as the conditions under which this reaction takes place.
Answer: Carbon dioxide reacts with hydrogen under certain conditions to form methanol. The reaction for the same is as follows: CO(g) + 2H2(g) → CH3OH Conditions for the reaction to take place:...
Choose the correct option.
A rectangular coil of copper wires is rotated in a magnetic field. The direction of the induced current changes once in each
a. two revolutions
b. one revolution
c. half revolution
d. one-fourth revolution
c. half revolution As we know that E.M.F is given by: E=nωBAcosωt On rotating a rectangular coil in a magnetic field, the direction of the induced current changes once per half revolution....
Write the balanced chemical equations for the following reactions, and add the state symbols:
(a) Magnesium carbonate reacts with hydrochloric acid to produce magnesium chloride, carbon dioxide, and water. (b) Sodium hydroxide reacts with sulphuric acid to produce sodium sulphate and water....
(c) Why is graphite a good conductor of electricity but diamond is a non-conductor of electricity?
(d) State any two uses of graphite. Solution: (c) Each carbon atom in a graphite crystal is linked to just three other carbon atoms. As a result, the fourth valence electron is free and is...
(a) What is graphite? Of what substance is graphite made?
(b) Describe the structure of graphite with the help of a labelled diagram. Solution: Solution: (a) Graphite is a carbon allotrope that is a soft, greyish black, and opaque carbon-based material....
Which sources produce alternating current?
Some sources that produce alternating current are: Power PlantsAC generator
(c) Explain why, diamond has a high melting point.
(d) State any two uses of a diamond. Solution: (c) It has a high melting point, and breaking the network of strong covalent connections requires a lot of energy. (d) (i) Used in the creation of...
Name some sources of direct current.
Some sources of direct current are: DC generatorsCells
State the principle of an electric generator.
The electromagnetic induction principle governs the operation of an electric generator. Electricity is generated in a generator by rotating a coil in a magnetic field.
(a) What is a diamond? Of what substance is a diamond made?
(b) Describe the structure of the diamond. Draw a simple diagram to show the arrangement of carbon atoms in a diamond. Solution: Solution: (a) A diamond is a clear, colourless material with...
Explain different ways to induce current in a coil.
The ways for inducing current in a coil are as follows: Electric current is induced in the coil when it is rapidly moved between the two poles of the horse shoe magnet.An electric current is induced...
(c) Explain why a diamond can be used in rock drilling equipment but graphite cannot.
(d) State one use of diamond which depends on its ‘extraordinary brilliance’ and one use of graphite that depends on its being ‘black and quite soft’. Solution: (c) Due to its hardness, diamond may...
(a) Write two points of difference in the structures of diamond and graphite.
(b) Explain why graphite can be used as a lubricant but diamond cannot. Solution: (a) (i) Carbon atoms in diamonds are bonded to four other carbon atoms. The carbon atom in graphite is connected to...
What is the role of split ring in an electric motor?
In an electric motor, the split ring serves as the commutator. After each half rotation of the coil, the commutator reverses the direction of the current flowing through it. The coil continues to...
What is the principle of an electric motor?
The magnetic effect of current is the basis for the operation of an electric motor. When put in a magnetic field, a current-carrying conductor experiences force and rotates. Fleming's Left-Hand Rule...
State Fleming’s left-hand rule.
The thumb, forefinger, and middle finger of the left hand are positioned at right angles according to Fleming's Left-Hand Rule. Then, The thumb will then point in the direction of magnetic force....
(a) Giving their structures, state the number of single bonds, double bonds and triple bonds(if any) following compounds:
(i) ethyne (ii) ethene (iii) benzene (b) Write the molecular formula and structure of cyclohexane. How many covalent bonds are there in the molecule of cyclohexane? Solution: (i)(ii) (iii) (i)...
(a) What is the unique property of a carbon atom? How is this property helpful to us?
(b) Explain why, diamond is hard while graphite is soft. Solution: (a) By joining atoms to atoms, carbon may create lengthy chains. The most distinctive feature of a carbon atom is catenation. As a...
A positively-charged particle (alpha-particle) projected towards the west is deflected towards north by a magnetic field. The direction of magnetic field is
a. towards south
b. towards east
c. downward
d. upward
The Fleming's Left hand rule can be used to determine the direction of the magnetic field. The thumb, forefinger, and middle finger of the left hand are right perpendicular to each other, therefore...
(b) Give the common name of (i) ethyne, and (ii) ethene.
(c) Write the molecular formula and structure of benzene. Solution: (b) (i) Acetylene (ii) Ethylene (c) C6H6
In Activity 13.7, how do we think the displacement of rod AB will be affected if length of the rod AB is increased?
In a magnetic field, a current-carrying conductor experiences force. The magnitude of this force grows as the amount of current, the length of the conductor and the strength of the magnetic field...
(a) Give the name and structural formula of one member each of the following:
(i) alkane ; (ii) alkene ; (iii) alkyne ; (iv) cycloalkane. Solution: (i)
In Activity 13.7, how do we think the displacement of rod AB will be affected if (i) current in rod AB is increased; (ii) a stronger horse-shoe magnet is used
In a magnetic field, a current-carrying conductor experiences force. The magnitude of this force grows as the amount of current, the length of the conductor, and the strength of the magnetic field...
(a) Why does the element carbon form a large number of carbon compounds?
(b) Write down the structures and names of two isomers of butane (C4H10). Solution: (a) Carbon can build long chains of carbon atoms through covalent bonding, which is why there are so many...
Which of the following property of a proton can change while it moves freely in a magnetic field? (There may be more than one correct answer.)
a. Mass
b. Speed
c. Velocity
d. Momentum
(c) and (d) When a proton enters a magnetic field region, it is subjected to magnetic force. As a result, the proton's journey becomes circular. The velocity and momentum change as a result.
(a) How can diamonds be made artificially? How do synthetic diamonds differ from natural ones?
(b) Give any two differences between the properties of diamond and graphite. What causes these differences? Solution: Diamonds may be created artificially by exposing pure carbon to extremely high...
The magnetic field inside a long straight solenoid-carrying current
a. is zero.
b. decreases as we move towards its end.
c. increases as we move towards its end.
d. is the same at all points.
d. is the same at all points Inside a long straight current carrying solenoid, the magnetic field is uniform, therefore it is the same at all places.
Catenation is the ability of an atom to form bonds with other atoms of the same element. It is exhibiting both carbon and silicon. Compare the ability of catenation of the two elements. Give reasons.
Solutions: An element's capacity to make bonds with other atoms of the same element is referred to as catenation. Both carbon and silicon show this property. In comparison to silicon, carbon has a...
a) Give IUPAC names and formulae of an organic compound containing single bonds and the other containing a triple bond.
(b) Which of the following is the molecular formula of benzene? C6H6 , C6H10 , C6H12 , C6H14 (c) Which of the two has a branched-chain: isobutene or normal butane? Solution: (a) Methane (single...
(a) Friedrich Wohler converted an inorganic compound into an organic compound in the laboratory.
(i) Give the name and formula of the inorganic compound. (ii) Write the name and formula of the organic compound formed. (b) Give the molecular formula of butane and mention the names of its two...
The magnetic field in a given region is uniform. Draw a diagram to represent it.
Consider a circular loop of wire lying in the plane of the table. Let the current pass through the loop clockwise. Apply the right-hand rule to find out the direction of the magnetic field inside and outside the loop.
The magnetic field will emerge from the table outside the loop and merge with the table inside the loop in the direction of the current flowing downhill. Similarly, when current flows upward, the...
Why don’t two magnetic field lines intersect each other?
If two magnetic field lines cross, the compass needle shows two different directions at the place of intersection, which is not feasible, hence they do not intersect.
List the properties of magnetic field lines.
The properties of magnetic field lines are: They do not intersect.They originate at the North Pole and conclude at the South Pole.The field lines inside the magnet are oriented from the South Pole...
Draw magnetic field lines around a bar magnet.
As seen in the diagram below, the magnetic field lines of a bar magnet arise from the North Pole and terminate at the South Pole.
Why does a compass needle get deflected when brought near a bar magnet?
A tiny magnet serves as the compass needle. When a compass needle is brought close to a bar magnet, the compass needle's magnetic field lines interact with the bar magnet's magnetic field lines....
Why does the sky appear dark instead of blue to an astronaut?
Because light scattering does not occur outside the earth's atmosphere, an astronaut sees the sky as dark rather than blue.
Why does the Sun appear reddish early in the morning?
Before reaching the observer, white light from the sun must travel a greater distance through the atmosphere. During this time, all coloured lights scatter save the light that corresponds to the red...
Explain why the planets do not twinkle?
Planets, unlike stars, do not sparkle. Stars are so far away that they appear in the night sky as pinpoints of light, even when viewed through a telescope. Because all of the light comes from a...
Why do stars twinkle?
The refraction of starlight being done by the atmosphere causes the twinkling of a star. When starlight enters the earth's atmosphere, it undergoes constant refraction before reaching the surface....
What happens to the image distance in the eye when we increase the distance of an object from the eye?
Even when an object is moved further away from the eye, an image is produced on the retina. As the object is pushed away from the eye, the eye lens grows smaller and the focus length rises.
Why is a normal eye not able to see clearly the objects placed closer than 25 cm?
Because the ciliary muscles of the eyes are unable to contract beyond a certain limit, a typical eye cannot perceive objects closer than 25 cm clearly.
Make a diagram to show how hypermetropia is corrected. The near point of a hypermetropic eye is 1 m. What is the power of the lens required to correct this defect? Assume that the near point of the normal eye is 25 cm.
Hypermetropia allows a person to see different items well, but also makes it difficult to perceive objects in close proximity. Because the eye lens focuses the incoming divergent rays beyond the...
(a) Aluminium hydroxide reacts with sulphuric acid to form aluminium sulphate and water. Write a balanced equation for this reaction. (b) Balance the following chemical equation: ![Rendered by QuickLaTeX.com \[\mathbf{Mn}{{\mathbf{O}}_{\mathbf{2}}}~+\text{ }\mathbf{HCl}~\to ~\mathbf{MnC}{{\mathbf{l}}_{\mathbf{2}}}~+\text{ }\mathbf{C}{{\mathbf{l}}_{\mathbf{2}}}~+\text{ }{{\mathbf{H}}_{\mathbf{2}}}\mathbf{O}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-317aa71deab1d49ce7504365db016a36_l3.png)
Answer: (a) 2Al(OH)3 + 3H2SO4 → Al2(SO4)3 + 6H2O (b) MnO2 + 4HCl → MnCl2 + Cl2 + 2H2O
Write any two observations in an activity that may suggest that a chemical reaction has taken place. Give an example in support of your answer
(i) Temperature shiftsWhen calcium oxide combines vigorously with water to generate calcium hydroxide, a considerable quantity of heat is released, causing the system's temperature to rise,...
(a) Explain, with example, how the physical states of the reactants and products can be shown in a chemical equation. (b) Balance the following equation and add state symbols: ![Rendered by QuickLaTeX.com \[\mathbf{Zn}\text{ }+\text{ }\mathbf{HCl}~\to ~\mathbf{ZnC}{{\mathbf{l}}_{\mathbf{2}}}~+\text{ }{{\mathbf{H}}_{\mathbf{2}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-0e9770d3c2ab9a6a471d45ce75a2df14_l3.png)
(c) Convey the following information in the form of a balanced chemical equation: “An aqueous solution of ferrous sulphate reacts with an aqueous solution of sodium hydroxide to form a precipitate of ferrous hydroxide and sodium sulphate remains in solution.”
Answer: (a) In a chemical reaction, the physical state of the reactants and products can be indicated by using the letters in the round brackets. If it is solid, it will be (s), liquid, it will be...
a) What is a balanced chemical equation? Why should chemical equations be balanced? b) Aluminium burns in chlorine to form aluminium chloride (AlCl3). Write a balanced chemical equation for this reaction. c) Potassium metal reacts with water to give potassium hydroxide and hydrogen gas. Write a balanced chemical equation for this reaction.
Answer: (a) By balancing the total number of atoms of each element in reactants and products, a chemical equation can be created. To follow the Law of Conservation of Mass, a chemical equation must...
The far point of a myopic person is 80 cm in front of the eye. What is the nature and power of the lens required to correct the problem?
Myopia is a condition that affects the individual. The image is created in front of the retina in this condition. As a result, a concave lens is utilized to treat this eyesight problem. Object...
(a) What are the various ways in which a chemical equation can be made more informative? Give examples to illustrate your answer. (b) Write a balanced chemical equation from the following information: An aqueous calcium hydroxide solution (lime water) reacts with carbon dioxide gas to produce a solid calcium carbonate precipitate and water.
Answer: (a) Ca(OH)2 (aq)+ CO2 (g)—3000°C →CaCO3(s)+H2O(l) A chemical equation can be informative by 1) The chemical states of substances present in the reaction are denoted by (s) for solids,...
When hydrogen is passed over copper oxide, copper and steam are formed. Write a balanced equation for this reaction and state which of the chemicals are: (i) Elements (ii) Compounds (iii) Reactants (iv) Products (v) Metals (vi) Non-metals
Answer: H2 + CuO→ Cu + H2O (i) H2 and Cu are elements (ii) CuO and H2O are compounds (iii) H2 and CuO are reactants (iv) Cu and H2O are products (v) Cu is the metal (vi) H2 is...
A person needs a lens of power -5.5 dioptres for correcting his distant vision. For correcting his near vision he needs a lens of power +1.5 dioptre. What is the focal length of the lens required for correcting (i) distant vision, and (ii) near vision?
The relation between the power and focal length is given by: Power (P) = 1/f (i) Power of the lens (used for correcting distant vision) as given is – 5.5 D Focal length of the lens (f) will...
(a) What is a chemical equation? Explain with the help of an example. (b) Giving examples, state the difference between balanced and unbalanced chemical equations. (c) Balance the following chemical equations: ![Rendered by QuickLaTeX.com \[~\left( \mathbf{i} \right)\text{ }\mathbf{N}{{\mathbf{H}}_{\mathbf{3}~}}\to \text{ }{{\mathbf{N}}_{\mathbf{2}~}}+\text{ }{{\mathbf{H}}_{\mathbf{2}}}\left( \mathbf{ii} \right)\text{ }\mathbf{C}\text{ }+\mathbf{C}{{\mathbf{O}}_{\mathbf{2}}}\to \text{ }\mathbf{CO}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-b15e1e600147f6c1f88738d016c15429_l3.png)
\
Solutions: Answer: (a) Chemical equations are symbols for chemical reactions in which the reactants and products are stated as chemical formulae. The left side of a chemical equation contains...
(a) Give one example of a chemical reaction. (b) State two characteristics of the chemical reaction which takes place when dilute sulphuric acid is poured over zinc granules. (c) Give two characteristics of the chemical reaction which occurs on adding potassium iodide solution to lead nitrate solution.
Answer: (a) By undergoing specific reactions and rearrangements, a chemical reaction produces new compounds. On the left, there will be reactants, and on the right, there will be products. The...
The least distance of distinct vision for a young adult with normal vision is about
(a) 25 m
(b) 2.5 cm
(c) 25 cm
(d) 2.5 m
(c) 25 cm For a young adult with normal vision, the minimum distance of clear vision is 25 cm.
The human eye forms an image of an object at its
(a) cornea
(b) iris
(c) pupil
(d) retina
(d) retina The retina is a layer of nerve cells that lines the interior of the eye's rear wall. This layer detects light and transmits information to the brain, allowing you to see.
The human eye can focus objects at different distances by adjusting the focal length of the eye lens. This is due to
(a) presbyopia
(b) accommodation
(c) near-sightedness
(d) far-sightedness
(b) accommodation The human eye can adapt the focal length of the eye lens to focus things at different distances because of accommodation.
Fill in the following blanks with suitable words: (a) Chemical equations are balanced to satisfy the law of _______. (b) A solution made in water is known as an ____ solution and indicated by the symbol _______.
Answer: (a) Conservation of mass (b) Aqueous; (aq)
A student has difficulty reading the blackboard while sitting in the last row. What could be the defect the child is suffering from? How can it be corrected?
Short-sightedness, often known as myopia, affects the student. Myopia can be rectified with the use of a power-appropriate concave or divergent lens.
Balance the following equations: ![Rendered by QuickLaTeX.com \[\left( \mathbf{i} \right)\text{ }\mathbf{A}{{\mathbf{l}}_{\mathbf{2}}}{{\left( \mathbf{SO4} \right)}_{\mathbf{3}}}~+\text{ }\mathbf{NaOH}\text{ }\to \text{ }\mathbf{Al}{{\left( \mathbf{OH} \right)}_{\mathbf{3}}}~+\text{ }\mathbf{N}{{\mathbf{a}}_{\mathbf{2}}}\mathbf{S}{{\mathbf{O}}_{\mathbf{4}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-47b921d974572c1df03bd3ca30daae59_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{ii} \right)\text{ }\mathbf{HN}{{\mathbf{O}}_{\mathbf{3}}}~+\mathbf{Ca}{{\left( \mathbf{OH} \right)}_{\mathbf{2}}}~\to \text{ }\mathbf{Ca}{{\left( \mathbf{N}{{\mathbf{O}}_{\mathbf{3}}} \right)}_{\mathbf{2}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-155af7574f93c010ccb69dad4596317c_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{iii} \right)\text{ }\mathbf{NaOH}\text{ }+{{\mathbf{H}}_{\mathbf{2}}}\mathbf{S}{{\mathbf{O}}_{\mathbf{4}}}~\to \text{ }\mathbf{N}{{\mathbf{a}}_{\mathbf{2}}}\mathbf{S}{{\mathbf{O}}_{\mathbf{4}}}~+{{\mathbf{H}}_{\mathbf{2}}}\mathbf{O}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-42e779e6cf6540f80b62788bedc5f8a8_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{iv} \right)\text{ }\mathbf{BaC}{{\mathbf{l}}_{\mathbf{2}}}~+\text{ }{{\mathbf{H}}_{\mathbf{2}}}\mathbf{S}{{\mathbf{O}}_{\mathbf{4}}}~\to \text{ }\mathbf{BaS}{{\mathbf{O}}_{\mathbf{4}}}~+\mathbf{HCl}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-ff9c89450bbae180e66877b92158435d_l3.png)
Answer: (i) (ii) (iii) (iv)
What is the far point and near point of the human eye with normal vision?
The near point of the eye is the shortest distance between an object and the eye at which it may be viewed clearly without strain. This distance is 25 cm for a regular person's eye. The far point of...
A person with a myopic eye cannot see objects beyond 1.2 m distinctly. What should be the type of corrective lens used to restore proper vision?
To restore adequate vision, a person with a myopic eye should use a concave lens with a focal length of 1.2 m.
What is meant by power of accommodation of the eye?
Power of accommodation of eye means: The ability of the eye's lens to change its focal length to clearly concentrate rays from far away as well as close objects on the retina.
Balance the following equations: ![Rendered by QuickLaTeX.com \[\left( \mathbf{i} \right)\text{ }\mathbf{Fe}\text{ }+{{\mathbf{O}}_{\mathbf{2}}}~\to \text{ }\mathbf{F}{{\mathbf{e}}_{\mathbf{2}}}{{\mathbf{O}}_{\mathbf{3}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-02c6afecd358f6421cfb1044b9cf319b_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{ii} \right)\text{ }\mathbf{Al}{{\left( \mathbf{OH} \right)}_{\mathbf{3}}}~\to \text{ }\mathbf{A}{{\mathbf{l}}_{\mathbf{2}}}{{\mathbf{O}}_{\mathbf{3}}}~+{{\mathbf{H}}_{\mathbf{2}}}\mathbf{O}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-56e1ce2cfa2bc8558eb869613b2a817d_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{iii} \right)\text{ }\mathbf{N}{{\mathbf{H}}_{\mathbf{3}}}+\text{ }\mathbf{CuO}\text{ }\to \text{ }\mathbf{Cu}\text{ }+{{\mathbf{N}}_{\mathbf{2}}}~+{{\mathbf{H}}_{\mathbf{2}}}\mathbf{O}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-0881654b71cf44d61dfb704ba4c11e0e_l3.png)
Answer: (i) (ii) (iii)
Balance the following equations: ![Rendered by QuickLaTeX.com \[\left( \mathbf{i} \right)\text{ }\mathbf{Na}\text{ }+\text{ }{{\mathbf{O}}_{\mathbf{2}}}~\to \text{ }\mathbf{N}{{\mathbf{a}}_{\mathbf{2}}}\mathbf{O}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-8714a7e5e31c46f0650ef10a3cd461ad_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{ii} \right)\text{ }{{\mathbf{H}}_{\mathbf{2}}}{{\mathbf{O}}_{\mathbf{2}}}\to \text{ }{{\mathbf{H}}_{\mathbf{2}}}\mathbf{O}\text{ }+\text{ }{{\mathbf{O}}_{\mathbf{2}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-217ecbe09eb1a5e807ce2367129ef46e_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{iii} \right)\text{ }\mathbf{Mg}{{\left( \mathbf{OH} \right)}_{\mathbf{2}}}~+\text{ }\mathbf{HCl}\text{ }\to \text{ }\mathbf{MgC}{{\mathbf{l}}_{\mathbf{2}}}~+{{\mathbf{H}}_{\mathbf{2}}}\mathbf{O}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-689a044051b673602fa040babfa9cac8_l3.png)
Answer:
What are the environmental consequences of the increasing demand for energy? What steps would you suggest to reduce energy consumption?
Industrialization demands more energy. To fulfil these demands, fossil fuels are used as they are readily available. It has an influence on the environment due to its rigorous use. Excessive usage...
Write complete balanced equations for the following reactions: (a) Calcium (solid) + Water (liquid) →Calcium hydroxide (solution) + Hydrogen (gas) (b) Sulphur dioxide (gas) + Oxygen (gas) → Sulphur trioxide (gas)
Answer:
Correct and balance the following equations: ![Rendered by QuickLaTeX.com \[\left( \mathbf{a} \right)~\mathbf{Ca}\text{ }+\text{ }{{\mathbf{H}}_{\mathbf{2}}}\mathbf{O}~\to ~\mathbf{CaOH}\text{ }+\text{ }\mathbf{H}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-1e64a2b6db2bc9e3e960957115665f7d_l3.png)
![Rendered by QuickLaTeX.com \[\left( \mathbf{b} \right)~\mathbf{N}\text{ }+\text{ }\mathbf{H}~\to ~\mathbf{N}{{\mathbf{H}}_{\mathbf{3}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-5da03fe9a6b5d23007e8dcbdd460d9c2_l3.png)
Answer: (a) (b)
Complete and balance the following equations:
(a) NaOH + ______ → Na2SO4 + H2O (b) Ca(OH)2 + _______ → CaCO3 + H2O Answer: (a) (b)
What are the advantages and disadvantages of using a solar cooker? Are there places where solar cookers would have limited utility?
Advantages are: A solar cooker gets its heat from the sun. It is a nonpolluting, renewable, and unlimited energy source. It will be cost-effective due to its endless supply. Disadvantages are:...
What are the qualities of an ideal source of energy?
The qualities of an ideal source of energy are: Is economicalIs easily availablePollution freeEasy to transport and storeAmount of energy produced on burning is huge.
Write the balanced chemical equations for the following reactions: (a) Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water (b) Aluminium + Copper chloride → Aluminium chloride + Copper
Answer: (a) (b)
Translate the following statements into chemical equations and then balance the equations: (a) Aluminium metal replaces iron from ferric oxide, Fe2O3, giving aluminium oxide and iron (b) Barium chloride reacts with zinc sulphate to give zinc chloride and barium sulphate.
Answer: (a) (b)
On what basis would you classify energy sources as:
a. Renewable and non-renewable?
b. Exhaustible and inexhaustible?Are the options given in (a) and (b) the same?
a) Renewable and non-renewable: Renewable energy sources are those that replenish themselves and are abundant in nature. Solar energy, tidal energy, wind energy, and bio-mass are examples....
Translate the following statements into chemical equations and then balance the equations: (a) Hydrogen sulphide gas burns in air to give water and sulphur dioxide. (b) Phosphorus burns in oxygen to give phosphorus pentoxide. (c) Carbon disulphate burns in the air to give carbon dioxide and sulphur dioxide.
Answer: Balanced chemical equations for the given reactions:-
What are the limitations of extracting energy from Tides?
To harvest energy from the tides, the sun, moon, and earth must be in perfect alignment, and the tides must be extremely strong.
How will you indicate the following effects in a chemical equation? (a) A solution made in water (b) Exothermic reaction (c) Endothermic reaction
Answer: (a) A water-based solution (b) Exothermic reaction (c) Thermodynamic reaction
What are the limitations of extracting energy from:
a. The wind?
b. Waves?
The wind: Windmills are used to capture wind energy. Windmills require a speed of more than 15 km/h to create electricity, which is one of the constraints of wind energy. And there will be more...
Compare and contrast bio-mass and hydroelectricity as sources of energy.
Biomass is derived from the decomposition of dead plants and animals. It is a renewable energy source. Wood and gobar-gas are examples of bio-mass energy sources. The potential energy of stored...
Why is photosynthesis considered an endothermic reaction?
The term "endothermic reaction" refers to a reaction in which heat is absorbed. As we all know, photosynthesis is a process in which green plants and other organisms use sunlight to convert carbon...
What does the symbol (aq) represent in a chemical equation?
The symbol (aq) represents an aqueous solution in a chemical equation.
What is wrong with the following chemical equation? Mg + O → MgO, correct and balance it.
Answer: Since oxygen is not present in its elemental form, so it is not possible for it to take part in the reaction as O. Here, Oxygen should be in molecular form, O2. The corrected and balanced...
State whether the following statement is true or false: A chemical equation can be balanced easily by altering the formula of a reactant or product.
Answer: False This statement is false because every reactant and product has a definite formula depending on the valency of the constituents. So, altering its formula for balancing an equation would...
Why should magnesium ribbon be cleaned before burning in the air?
Magnesium ribbon is made of pure magnesium metal. It is highly reactive with oxygen, resulting in the oxidation of magnesium when it comes into contact with air. It produces an oxide layer on the...
What happens chemically when quicklime is added to water filled in a bucket?
Calcium oxide is the chemical formula for quick lime. When calcium hydroxide (slaked lime) is introduced to water in a bucket, it forms calcium hydroxide (slaked lime) and reacts rapidly, producing...
On what basis is a chemical equation balanced?
A chemical equation is always balanced when the number of atoms of each element on the reactant and product sides is compared. It is based on the Law of Conservation of Mass, which states that the...
Why is respiration considered an exothermic process?
An exothermic reaction is the one in which energy is released. As energy is released during the process of breathing, it is referred to as an exothermic reaction.
Several electric bulbs designed to be used on a 220 V electric supply line, are rated 10 W. How many lamps can be connected in parallel with each other across the two wires of 220 V line if the maximum allowable current is 5 A?
The bulb's resistance may be estimated using the formula. V2/R1 = P1 V2/P1 = R1 We obtain by substituting the values. x=110 As a result, 110 lights may be linked in series.
Show how you would connect three resistors, each of resistance 6 Ω, so that the combination has a resistance of (i) 9 Ω, (ii) 4 Ω.
When all three resistors are connected in series, the equivalent resistance is 6 + 6 + 6 =18, which is not the required number. Similarly, if all three resistors are connected in parallel, the...
How many 176 Ω resistors (in parallel) are required to carry 5 A on a 220 V line?
Let's call the needed number of resistors 'x.' The parallel combination of resistors R's equivalent resistance is given by Using ohms law, find the no of resisters. A total of four resistors are...
A battery of 9 V is connected in series with resistors of 0.2 Ω, 0.3 Ω, 0.4 Ω, 0.5 Ω and 12 Ω, respectively. How much current would flow through the 12 Ω resistor?
There is no present division in a series link. All of the resistors have the same current flowing through them. Ohm's law is used to compute the amount of current flowing across the resistors. But...
When a 12 V battery is connected across an unknown resistor, there is a current of 2.5 mA in the circuit. Find the value of the resistance of the resistor
Using Ohm's Law, the value of the resistance may be determined as follows: R=4.8k ohms
The values of current I flowing in a given resistor for the corresponding values of potential difference V across the resistor are given below –
Plot a graph between V and I and calculate the resistance of that resistor.
The IV characteristic is a graph that shows the relationship between voltage and current. On the y-axis, the current is shown, while the voltage is represented on the x-axis. The table below shows...
A copper wire has diameter 0.5 mm and resistivity of  Ω m. What will be the length of this wire to make its resistance 10 Ω? How much does the resistance change if the diameter is doubled?
Ω m. What will be the length of this wire to make its resistance 10 Ω? How much does the resistance change if the diameter is doubled?
The resistance of a copper wire with a length in metres and a cross-sectional area of metre sq. is calculated using the formula. R=\(\rho\)\/A On substituting the values we get: The wire is 122.72...
How is a voltmeter connected in the circuit to measure the potential difference between two points?
The voltmeter should be connected in parallel between the two locations to measure the voltage between them.
Two conducting wires of the same material and of equal lengths and equal diameters are first connected in series and then parallel in a circuit across the same potential difference. The ratio of heat produced in series and parallel combinations would be _____.
(a) 1:2
(b) 2:1
(c) 1:4
(d) 4:1
(c) 1:4
An electric bulb is rated 220 V and 100 W. When it is operated on 110 V, the power consumed will be _____.
(a) 100 W
(b) 75 W
(c) 50 W
(d) 25 W
(d) 25 W Step 1: Using the above ratings, determine the bulb's resistance. Bulb rating: P=100W, V=220V Power, $P=\frac{V^{2}}{R}$$\Rightarrow \mathrm{R}=\frac{\mathrm{V}^{2}}{\mathrm{P}}=\frac{220...
Which of the following does not represent electrical power in a circuit?
(a) 
(b) 
(c) VI
(d) /R
/R
(b) \(I{{R}^{2}}\)
A piece of wire of resistance R is cut into five equal parts. These parts are then connected in parallel. If the equivalent resistance of this combination is R′, then the ratio R/R′ is _____.
(a) 1/25
(b) 1/5
(c) 5
(d) 25
d) 25 We know that. $\text { Resistance } \mathrm{R}=\rho \frac{\ell}{\mathrm{A}}$ Because R is proportional to the length of the wire.As a result, after breaking the wire into five sections, the...
An electric motor takes 5 A from a 220 V line. Determine the power of the motor and the energy consumed in 2 h.
The equation may be used to compute the motor's power. VI = P We obtain by substituting the numbers in the preceding equation. 220 V x 5 A = 1100 W The equation may be used to calculate the motor's...
What determines the rate at which energy is delivered by a current?
Electric power is the pace at which electrical energy is used by electric equipment. As a result, the power of an appliance is defined as the rate at which energy is provided by a current.
An electric iron of resistance 20 Ω takes a current of 5 A. Calculate the heat developed in 30 s.
The Joule's law of heating, which is provided by the equation, may be used to compute the quantity of heat created. VIt = H We obtain by substituting the variables in the preceding equation: H =...
Compute the heat generated while transferring 96000 coulomb of charge in one hour through a potential difference of 50 V.
Joule's law may be used to calculate the amount of heat produced: VIt = H where, V = 50 V is the voltage. I is the current. 1 hour Equals 3600 seconds, where t is the time in seconds. The following...
Why does the cord of an electric heater not glow while the heating element does?
The heating element of an electric heater is composed of a high-resistance alloy. When the current passes through the heating element, it becomes overheated and glows red. The cord is generally...
What is (a) the highest, (b) the lowest total resistance that can be secured by combinations of four coils of resistance 4 Ω, 8 Ω, 12 Ω, 24 Ω?
(a) When the four resistors are wired in series, their total resistance equals the sum of their individual resistances, which is the greatest. 4 + 8 + 12 + 24 = 48 is the total equivalent resistance...
