Despite the fact that $H e_{2}^{3}$ and $H e_{1}^{3}$ have the same mass number, their binding energies are different. Because the number of protons and neutrons in both nuclei differs, the binding...
Is the given relation a function? Give reasons for your answer. (i) g = n, (1/n) |n is a positive integer (ii) s = {(n, n2) | n is a positive integer}
Solution: (i) Provided, $\mathrm{g}=\mathrm{n},(1 / \mathrm{n}) \mid \mathrm{n}$ is a positive integer As a result, the element n is a positive integer and the corresponding 1/n will be a unique and...
Is the given relation a function? Give reasons for your answer. (i) h = {(4, 6), (3, 9), (– 11, 6), (3, 11)} (ii) f = {(x, x) | x is a real number}
Solution: (i) Provided, h = {(4, 6), (3, 9), (– 11, 6), (3, 11)} Hence, element 3 has two images, namely, 9 and 11. If every element of one set has one and only one image in other set then a...
Samples of two radioactive nuclides A and B are taken. 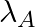 and
and 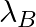 are the disintegration constants of A and B respectively. In which of the following cases, the two samples can simultaneously have the same decay rate at any time?
are the disintegration constants of A and B respectively. In which of the following cases, the two samples can simultaneously have the same decay rate at any time?
(a) Initial rate of decay of A is twice the initial rate of decay of B and 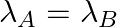
(b) Initial rate of decay of A is twice the initial rate of decay of B and 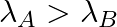
(c) Initial rate of decay of B is twice the initial rate of decay of A and 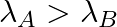
(d) Initial rate of decay of B is the same as the rate of decay of A at 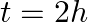 and
and 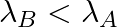
The correct options are: (b) Initial rate of decay of A is twice the initial rate of decay of B and $\lambda _A>\lambda_B$ (d) Initial rate of decay of B is the same as the rate of decay of A at...
In a nuclear reactor, moderators slow down the neutrons which come out in a fission process. The moderator used to have light nuclei. Heavy nuclei will not serve the purpose because
(a) they will break up
(b) elastic collision of neutrons with heavy nuclei will not slow them down
(c) the net weight of the reactor would be unbearably high
(d) substances with heavy nuclei do not occur in a liquid or gaseous state at room temperature
The correct option is: (b) elastic collision of neutrons with heavy nuclei will not slow them down
Tritium is an isotope of hydrogen whose nucleus Triton contains 2 neutrons and 1 proton. Free neutrons decay into 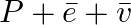 . If one of the neutrons in Triton decays, it would transform into He3 nucleus. This does not happen. This is because
. If one of the neutrons in Triton decays, it would transform into He3 nucleus. This does not happen. This is because
(a) Triton energy is less than that of a 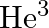 nucleus
nucleus
(b) the electron created in the beta decay process cannot remain in the nucleus
(c) both the neutrons in triton have to decay simultaneously resulting in a nucleus with 3 protons, which is not a 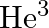 nucleus
nucleus
(d) because free neutrons decay due to external perturbations which is absent in a triton nucleus
The correct option is: (a) Triton energy is less than that of a $\mathrm{He}^{3}$ nucleus
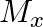 and
and 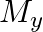 denote the atomic masses of the parent and the daughter nuclei respectively in a radioactive decay. The Q-value for a
denote the atomic masses of the parent and the daughter nuclei respectively in a radioactive decay. The Q-value for a 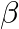 – decay is
– decay is 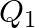 and that for a
and that for a 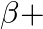 decay is
decay is 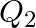 . If me denotes the mass of an electron, then which of the following statements is correct?
. If me denotes the mass of an electron, then which of the following statements is correct?
(a) 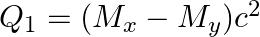 and
and 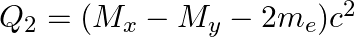
(b) 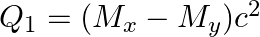 and
and 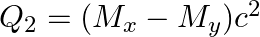
(c) 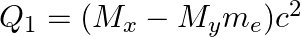 and
and 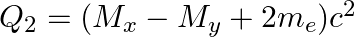
(d) 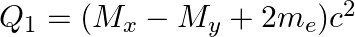 and
and 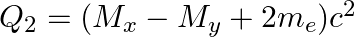
The correct option is: (a) $Q_ 1=(M _x-M_ y) c^{2}$ and $Q_ 2=(M _x-M_ y-2 m_ e) c^{2}$
If 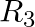 = {(x, |x| ) |x is a real number} is a relation. Then find domain and range of
= {(x, |x| ) |x is a real number} is a relation. Then find domain and range of 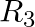 .
.
Solution: Provided, $\mathrm{R}_3=\{(\mathrm{x},|\mathrm{x}|) \mid \mathrm{x}$ is a real number $\}$ is a relation All the first elements of all the ordered pairs of R3, i.e., x, are included in...
When a nucleus in an atom undergoes radioactive decay, the electronic energy levels of the atom
(a) do not change for any type of radioactivity
(b) change for α and β radioactivity but not for γ-radioactivity
(c) change for α-radioactivity but not for others
(d) change for β-radioactivity but not for others
The correct option is: (b) change for α and β radioactivity but not for γ-radioactivity
If 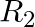 = {(x, y) | x and y are integers and x2 + y2 = 64} is a relation. Then find
= {(x, y) | x and y are integers and x2 + y2 = 64} is a relation. Then find 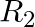 .
.
Solution: Given, $\mathrm{R}_2=\{(\mathrm{x}, \mathrm{y})$ x and y are integers and $\left.x^{2}+y^{2}-64\right\}$ As a result, we have, $x^{2} = 0$ and $y^{2} = 64$ or $x^{2} = 64$ and $y^{2} = 0$...
Suppose we consider a large number of containers each containing initially 10000 atoms of a radioactive material with a half-life of 1 year. After 1 year
(a) all the containers will have 5000 atoms of the material
(b) all the containers will contain the same number of atoms of the material but that number will only be approximately 5000
(c) the containers will, in general, have different numbers of the atoms of the material but their average will be close to 5000
(d) none of the containers can have more than 5000 atoms
The correct option is: (c) the containers will, in general, have different numbers of the atoms of the material but their average will be close to 5000
If 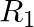 = {(x, y) | y = 2x + 7, where x ∈ R and – 5 ≤ x ≤ 5} is a relation. Then find the domain and Range of
= {(x, y) | y = 2x + 7, where x ∈ R and – 5 ≤ x ≤ 5} is a relation. Then find the domain and Range of 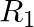 .
.
Solution: Provided, $R_1$ $=\{(x, y) \mid y=2 x+7$, in which x ∈R and ${-} 5\leq x\leq 5}$ is a relation All the first elements of all the ordered pairs of $R_1$, i.e., x, are included in the domain...
Given R = {(x, y) : x, y ∈ W, x2 + y2 = 25}. Find the domain and Range of R.
Solution: Provided, R $=\left\{(x, y): x, y \in W,x^{2}+y^{2}=25\right\}$ R $=\{(0,5),(3,4),(4,3),(5,0)\}$ R's domain consists of all the first elements of all the ordered pairs of R. Domain of R...
Given A = {1, 2, 3, 4, 5}, S = {(x, y) : x ∈ A, y ∈ A}. Find the ordered pairs which satisfy the conditions given below: x + y > 8
x + y > 8 Finding the ordered pair such that x + y>8, where x and y belong to the given set A: 1 + 1 = 2<8 1 + 2 = 3<8 1 + 3 = 4<8 1 + 4 = 5<8 1 + 5 = 6<8 2 + 1 = 3<8 2 + 2 =...
Suppose 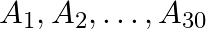 are thirty sets each having 5 elements and
are thirty sets each having 5 elements and 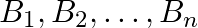 are
are 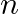 sets each with 3 elements, let
sets each with 3 elements, let 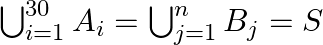 and each element of
and each element of 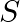 belongs to exactly 10 of the
belongs to exactly 10 of the 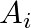 ‘s and exactly 9 of the B,’S. then
‘s and exactly 9 of the B,’S. then 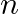 is equal to A. 15 B. 3 C. 45 D. 35
is equal to A. 15 B. 3 C. 45 D. 35
Solution: As per the question, $\bigcup_{i=1}^{30} A_{i}=\bigcup_{j=1}^{n} B_{j}=S$ As the elements are not repeating, no. of elements in $\mathrm{A}_{1} \cup \mathrm{A}_{2} \cup \mathrm{A}_{3} \cup...
Given A = {1, 2, 3, 4, 5}, S = {(x, y) : x ∈ A, y ∈ A}. Find the ordered pairs which satisfy the conditions given below:(i) x + y = 5(ii) x + y < 5
Given, A = {1, 2, 3, 4, 5}, S = {(x, y) : x ∈A, y ∈A} (i) x + y = 5 Finding the ordered pair such that x + y = 5, where x and y belong to the given set A: 1 + 1 = 2≠5 1 + 2 = 3≠5 1 + 3 = 4≠5 1 + 4 =...
In a group of 50 students, the number of students studying French, English, Sanskrit were found to be as follows: French = 17, English = 13, Sanskrit = 15 French and English = 09, English and Sanskrit = 4 French and Sanskrit = 5, English, French and Sanskrit = 3. Find the number of students who study (i) at least one of the three languages (ii) none of the three languages
Solution: Given, $50 =$ Total number of students $13 =$ Number of students studying English $17 =$ Number of students studying French $15 =$ Number of students studying Sanskrit $9 =$ Number of...
In a group of 50 students, the number of students studying French, English, Sanskrit were found to be as follows: French = 17, English = 13, Sanskrit = 15 French and English = 09, English and Sanskrit = 4 French and Sanskrit = 5, English, French and Sanskrit = 3. Find the number of students who study (i) French and Sanskrit but not English (ii) French and English but not Sanskrit
Solution: Given, $50 =$ Total number of students $13 =$ Number of students studying English $17 =$ Number of students studying French $15 =$ Number of students studying Sanskrit $9 =$ Number of...
In a group of 50 students, the number of students studying French, English, Sanskrit were found to be as follows: French = 17, English = 13, Sanskrit = 15 French and English = 09, English and Sanskrit = 4 French and Sanskrit = 5, English, French and Sanskrit = 3. Find the number of students who study (i) Sanskrit only (ii) English and Sanskrit but not French
Solution: Given, $50 =$ Total number of students $13 =$ Number of students studying English $17 =$ Number of students studying French $15 =$ Number of students studying Sanskrit $9 =$ Number of...
In a group of 50 students, the number of students studying French, English, Sanskrit were found to be as follows: French = 17, English = 13, Sanskrit = 15 French and English = 09, English and Sanskrit = 4 French and Sanskrit = 5, English, French and Sanskrit = 3. Find the number of students who study (i) French only (ii) English only
Solution: Given, $50 =$ Total number of students $13 =$ Number of students studying English $17 =$ Number of students studying French $15 =$ Number of students studying Sanskrit $9 =$ Number of...
In a town of 10,000 families it was found that 40% families buy newspaper A, 20% families buy newspaper B, 10% families buy newspaper C, 5% families buy A and B, 3% buy B and C and 4% buy A and C. If 2% families buy all the three newspapers. Find (a) The number of families which buy newspaper A only. (b) The number of families which buy none of A, B and C
Solution: As per the question, $=10,000$ = Total number of families $\mathrm{A}=\mathrm{n}(\mathrm{A})=40 \%$ = Number of families buying newspaper $B=n(B)=20 \%$ = Number of families buying...
In each of the following cases, find a and b. (i) (2a + b, a – b) = (8, 3) (ii) (a/4 , a – 2b) = (0, 6 + b)
(i) Given, (2a + b, a – b) = (8, 3) The corresponding elements will be equal because the ordered pairs are equal. Hence, 2a + b = 8 and a–b = 3 Now a–b = 3 ⇒a = 3 + b On putting the value of a in...
If P = {x : x < 3, x ∈ N}, Q = {x : x ≤ 2, x ∈ W}. Find (P ∪ Q) × (P ∩ Q), where W is the set of whole numbers.
Given, P = {x: x < 3, x ∈N}, Q = {x : x ≤ 2, x ∈W} where W is the set of whole numbers P = {1, 2} Q = {0, 1, 2} Now (P∪Q) = {1, 2}∪{0, 1, 2} = {0, 1, 2} And, (P∩Q) = {1, 2}∩{0, 1, 2} = {1, 2}...
Let A = {–1, 2, 3} and B = {1, 3}. Determine i) A × B(ii) B × A
Given, A = {–1, 2, 3} and B = {1, 3} (i) A × B {–1, 2, 3} × {1, 3} So, A × B = {(–1, 1), (–1, 3), (2, 1), (2, 3), (3, 1), (3, 3)} As a result, the Cartesian product is {(–1, 1), (–1, 3), (2, 1), (2,...
In a survey of 200 students of a school, it was found that 120 study Mathematics, 90 study Physics and 70 study Chemistry, 40 study Mathematics and Physics, 30 study Physics and Chemistry, 50 study Chemistry and Mathematics and 20 none of these subjects. Find the number of students who study all the three subjects.
Solution: As per the question, n(U) = 200 = Total number of students n(M) = 120 = Number of students who study Mathematics n(P) = 90 = Number of students who study Physics n(C) = 70 = Number of...
In a class of 60 students, 25 students play cricket and 20 students play tennis, and 10 students play both the games. Find the number of students who play neither?
Solution: As per the question, 60 = Total number of students 25 = Students who play cricket 20 = Students who play tennis 10 = Students who play both the games We need to find: the number of...
Out of 100 students; 15 passed in English, 12 passed in Mathematics, 8 in Science, 6 in English and Mathematics, 7 in Mathematics and Science; 4 in English and Science; 4 in all the three. Find how many passed (iii) in Mathematics only (iv) in more than one subject only.
Solution: As per the question, 100 = Total number of students 15 = Number of students who passed in English 12 = Number of students who passed in Mathematics 8 = Number of students who passed in...
Out of 100 students; 15 passed in English, 12 passed in Mathematics, 8 in Science, 6 in English and Mathematics, 7 in Mathematics and Science; 4 in English and Science; 4 in all the three. Find how many passed (i) in English and Mathematics but not in Science (ii) in Mathematics and Science but not in English
Solution: As per the question, 100 = Total number of students 15 = Number of students who passed in English 12 = Number of students who passed in Mathematics 8 = Number of students who passed in...
Let A, B and C be sets. Then show that A ∩ (B ∪ C) = (A ∩ B) ∪ (A ∩ C)
Solution: As per the question The three given sets are A, B and C We need to prove: $A \cap(B \cup C)=(A \cap B) \cup(A \cap C)$ Let's say $x \in A \cap(B \cup C)$ $\Rightarrow x \in A$ and $x \in(B...
Using properties of sets prove the statements given in Exercise let,T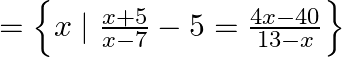 Is T an empty set? Justify your answer.
Is T an empty set? Justify your answer.
Solution: As per the question T$=\left\{x \mid \frac{x+5}{x-7}-5=\frac{4 x-40}{13-x}\right\}$ We need to check whether T is an empty set or not, Now solve, $\frac{x+5}{x-7}-5=\frac{4 x-40}{13-x}$...
Using properties of sets prove the statements given in Exercise for all sets A and B, (A ∪ B) – B = A – B
Solution: As per the question, Two sets are A and B We need to prove: $(A \cup B)-B=A-B$ Left Hand Side $=(A \cup B)-B$ As, $A-B=A \cap B^{\prime}$, we have, $=(A \cup B) \cap B^{\prime}$ As,...
Using properties of sets prove the statements given in Exercise for all sets A and B, A – (A ∩ B) = A – B
Solution: As per the question, Two sets A and B We need to prove: $A-(A \cap B)=A-B$ Left Hand Side =$\mathrm{A}-(\mathrm{A} \cap \mathrm{B})$ As, $A-B=A \cap B^{\prime}$, we have, $=A \cap(A \cap...
Using properties of sets prove the statements given in Exercise for all sets A and B, A – (A – B) = A ∩ B.
Solution: As per the question, Two sets are $A$ and $B$ We need to prove: $A-(A-B)=A \cap B$ Left Hand Side =$\mathrm{A}-(\mathrm{A}-\mathrm{B})$ As, $A-B=A \cap B^{\prime}$, we have,...
Using properties of sets prove the statements given in Exercise for all sets A and B, A ∪ (B – A) = A ∪ B.
Solution: As per the question, Two sets A and B We need to prove: $A \cup(B-A)=A \cup B$ Left Hand Side $=A \cup(B-A)$ As, $A-B=A \cap B^{\prime}$, we have, $=A \cup\left(B \cap A^{\prime}\right)$...
Determine whether each of the statement in Exercise for all sets A, B and C, if A ⊂ C and B ⊂ C, then A ∪ B ⊂ C, is true or false. Justify your answer.
Solution: The statement is true As per the question, Three sets are A, B and C We need to check: if $A \subset C$ and $B \subset C$, therefore $A \cup B \subset C$ is true or false Let's say $x \in...
Determine whether each of the statement in Exercise for all sets A, B and C, if A ⊂ B, then A ∪ C ⊂ B ∪ C, is true or false. Justify your answer.
Solution: The statement is true As per the question, Three sets are A, B and C We need to check: if $A \subset B$, therefore $A \cup C \subset B \cup C$ is true or false Let's say $x \in A \cup C$...
Determine whether each of the statement in Exercise for all sets A, B and C, if A ⊂ B, then A ∩ C ⊂ B ∩ C, is true or false. Justify your answer.
Solution: The statement is true As per the question, Three sets are A, B and C We need to check: if $A \subset B$, therefore $A \cap C \subset B \cap C$ is true or false Let's say $x \in A \cap C$...
Determine whether each of the statement in Exercise for all sets A, B and C, A – (B – C) = (A – B) – C, is true or false. Justify your answer.
Solution: The statement is false As per the question, Three sets are A, B and C We need to check: $A-(B-C)=(A-B)-C$ is true or false. Step 1: $B-C$ Step 2: $A-(B-C)$ Step 3: $A-B$ Step 4:...
A plane EM wave travelling along z-direction is described by 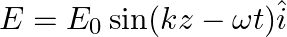 and
and 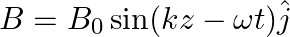 Show that
Show that
i) the average energy density of the wave is given by 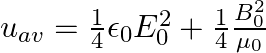
ii) the time-averaged intensity of the wave is given by 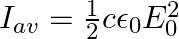
i) The energy density due to electric field $E$ is given as $\mathrm{uE}=1 / 2 \varepsilon_{0} \mathrm{E}^{2}$ The energy density due to magnetic field $B$ is givena as $\mathrm{uB}=1 / 2...
Determine whether each of the statement in Exercise For all sets A and B, (A – B) ∪ (A ∩ B) = A is true or false. Justify your answer.
Solution: The statement is true As per the question, Two sets A and B We need to check: $(A-B) \cup(A \cap B)=A$ is true or false Left Hand Side $=(A-B) \cup(A \cap B)$ As, $A-B=A \cap B'$ , We...
A plane EM wave travelling in vacuum along z-direction is given by 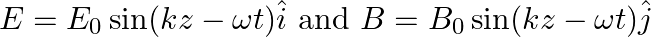
a) Use equation 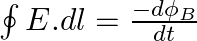 to prove
to prove 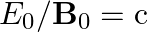
b) by using a similar process and the equation 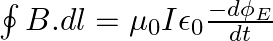 , prove that
, prove that 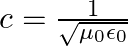
a) Substituting the above equations in the following equation we get ${c} \oint E . d l=-\frac{d \phi_{B}}{d t}=-\frac{d}{d t} \oint B \cdot d s$ So, $E_{0} / B_{0}=0$ b) We get $c=1 /...
For all sets A, B and C, show that (A – B) ∩ (A – C) = A – (B ∪ C)
Solution: As per the question, A, B and C are three sets We need to show: $(\mathrm{A}-\mathrm{B}) \cap(\mathrm{A}-\mathrm{C})=\mathrm{A}-(\mathrm{B} \cup \mathrm{C})$ $\text { Let's say }...
A long straight cable of length 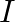 is placed symmetrically along the z-axis and has radius
is placed symmetrically along the z-axis and has radius 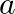 . The cable consists of a thin wire and a co-axial conducting tube. An alternating current
. The cable consists of a thin wire and a co-axial conducting tube. An alternating current 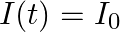 sin
sin 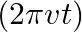 flows down the central thin wire and returns along the co-axial conducting tube. The induced electric field at a distance
flows down the central thin wire and returns along the co-axial conducting tube. The induced electric field at a distance 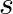 from the wire inside the cable is
from the wire inside the cable is 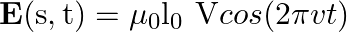 . In
. In  ,
,
compare the conduction current 10 with the displacement current 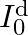
The displacement will be, $I_{0}^{\mathrm{d}} / \mathrm{I}_{0}=(\mathrm{am} / \lambda)^{2}$
Show that the following system of linear inequalities has no solution x + 2y ≤ 3, 3x + 4y ≥ 12, x ≥ 0, y ≥ 1
SOLUTION: \[\begin{array}{*{35}{l}} x\text{ }+\text{ }2y\text{ }\le \text{ }3 \\ {} \\ \end{array}\] \[Line:\text{ }x\text{ }+\text{ }2y\text{ }=\text{ }3\] x 3 1 y 0 1 Also, (0, 0) satisfies...
Seawater at frequency 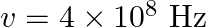 has permittivity
has permittivity 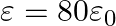 , permeability
, permeability 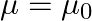 and resistivity
and resistivity 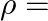
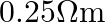 . Imagine a parallel plate capacitor immersed in seawater and driven by an alternating voltage source
. Imagine a parallel plate capacitor immersed in seawater and driven by an alternating voltage source 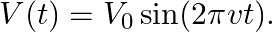 What fraction of the conduction current density is the displacement current density?
What fraction of the conduction current density is the displacement current density?
The separation between the plates of the capacitor is given as $V(t)=V_{0} \sin (2 \pi v t)$ Ohm's law for the conduction of current density is given as $\mathrm{J}_{0}{...
Let U be the set of all boys and girls in a school, G be the set of all girls in the school, B be the set of all boys in the school, and S be the set of all students in the school who take swimming. Some, but not all, students in the school take swimming. Draw a Venn diagram showing one of the possible interrelationship among sets U, G, B and S.
Solution: As per the question, Four sets given here are U, G, B, S So here, A universal set containing set of all boys and girls in a school = U Set of all girls in the school = G Set of all boys in...
An infinitely long thin wire carrying a uniform linear static charge density 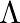 is placed along the z-axis. The wire is set into motion along its length with a uniform velocity
is placed along the z-axis. The wire is set into motion along its length with a uniform velocity 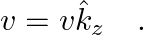 Calculate the pointing vectors
Calculate the pointing vectors 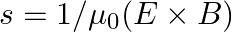
The electric field in an infinitely long thin wire is given by the expression, $\vec{E}=\frac{\lambda \hat{e}_{s}}{2 \pi \epsilon_{0} a} \hat{j}$ Magnetic field due to the wire is given by the...
A, B and C are subsets of Universal Set U. If A = {2, 4, 6, 8, 12, 20}, B = {3, 6, 9, 12, 15}, C = {5, 10, 15, 20} and U is the set of all whole numbers, draw a Venn diagram showing the relation of U, A, B and C.
Solution: As per the question, $A=\{2,4,6,8,12,20\}, B=\{3,6,9,12,15\}, C=\{5,10,15,20\}$ U is a universal set here Hence, $\begin{array}{l} \Rightarrow \mathrm{A} \cap \mathrm{B}=\{2,4,6,8,12,20\}...
Find the linear inequalities for which the shaded region in the given figure is the solution set.
SOLUTION: Considering \[x\text{ }+\text{ }y\text{ }=\text{ }8,\] The shaded region and the origin both are on the same side of the graph of the line and (0, 0) satisfy the constraint \[x\text{...
Find the linear inequalities for which the shaded region in the given figure is the solution set.
SOLUTION: According to the question, Considering \[3x\text{ }+\text{ }2y\text{ }=\text{ }48,\] The shaded region and the origin both are on the same side of the graph of the line and (0, 0) satisfy...
Solve the following system of inequalities
Solution: For above fraction be greater than 0, either both denominator and numerator should be greater than 0 or both should be less than 0. \[\begin{array}{*{35}{l}} \Rightarrow ~6\text{ }\text{...
In drilling world’s deepest hole it was found that the temperature T in degree Celsius, x km below the earth’s surface was given by T = 30 + 25 (x – 3), 3 ≤ x ≤ 15. At what depth will the temperature be between 155°C and 205°C?
\[T\text{ }=\text{ }30\text{ }+\text{ }25\left( x\text{ }\text{ }3 \right),\text{ }3\text{ }\le \text{ }x\text{ }\le \text{ }15;\] where, T = temperature and x = depth inside the earth The...
The longest side of a triangle is twice the shortest side and the third side is 2 cm longer than the shortest side. If the perimeter of the triangle is more than 166 cm then find the minimum length of the shortest side.
Let the length of shortest side = ‘x’ cm According to the question, The longest side of a triangle is twice the shortest side ⇒ Length of largest side = 2x Also, the third side is 2 cm longer than...
A solution is to be kept between 40°C and 45°C. What is the range of temperature in degree Fahrenheit, if the conversion formula is F = 9/5 C + 32?
Let temperature in Celsius be C Let temperature in Fahrenheit be F According to the question, Solution should be kept between 40° C and 45°C ⇒ 40 < C < 45 Multiplying each term by 9/5, we get...
Even though an electric field E exerts a force qE on a charged particle yet the electric field of an EM wave does not contribute to the radiation pressure. Explain.
Despite the fact that an electric field E imposes a force qE on a charged particle, the electric field of an EM wave has no effect on radiation pressure because radiation pressure is the product of...
A solution of 9% acid is to be diluted by adding 3% acid solution to it. The resulting mixture is to be more than 5% but less than 7% acid. If there is 460 litres of the 9% solution, how many litres of 3% solution will have to be added?
According to the question, Let x litres of 3% solution is to be added to 460 liters of the 9% of solution Then, we get, Total solution = \[\left( 460\text{ }+\text{ }x \right)\text{ }litres\] Total...
Show that the radiation pressure exerted by an EM wave of intensity I on a surface kept in vacuum is I/c.
Energy received by the surface per second is given as $E = IA$ Number of photons received by the surface per second is given as $N$ The perfect absorbing can be expressed as $h/ \lambda$ Hence,...
The water acidity in a pool is considered normal when the average pH reading of three daily measurements is between 8.2 and 8.5. If the first two pH readings are 8.48 and 8.35, find the range of pH value for the third reading that will result in the acidity level being normal.
According to the question, First reading = 8.48 Second reading = 8.35 Now, let the third reading be ‘x’ Average pH should be between 8.2 and 8.5 Average \[pH~=\text{ }\left( 8.48\text{ }+\text{...
You are given a 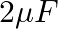 parallel plate capacitor. How would you establish an instantaneous displacement current of
parallel plate capacitor. How would you establish an instantaneous displacement current of 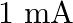 in the space between its plates?
in the space between its plates?
The capacitance of the capacitor is given by $\mathrm{C}=2 \mu \mathrm{F}$ Displacement current is given as $I_{d}=1 \mathrm{~mA}$ Hence, Charge in capacitor will be, $q=C V$ $\mathrm{dV} /...
Since, Profit = Revenue – cost Requirement is, profit > 0 \[C\left( x \right)\text{ }=\text{ }26,000\text{ }+\text{ }30\text{ }x;\] where x is number of cassettes \[\begin{array}{*{35}{l}}...
Show that the average value of radiant flux density S over a single period 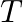 is given by
is given by 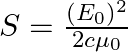
Radiant flux density is given as $\vec{S}=\frac{1}{\mu_{0}}\left(\vec{E} \times \vec B\right)=c^{2} \epsilon_{0}\left({\vec{E}} \times \vec B\right)$ $\mathrm{E}=\mathrm{E}_{0} \cos...
Solve for x, the inequalities in 4x + 3 ≥ 2x + 17, 3x – 5 < – 2.
\[\begin{array}{*{35}{l}} 4x\text{ }+\text{ }3\text{ }\ge \text{ }2x\text{ }+\text{ }17 \\ \Rightarrow ~4x\text{ }\text{ }-2x\text{ }\ge \text{ }17\text{ }\text{ }-3 \\ \Rightarrow ~2x\text{ }\ge...
Solve for x, the inequalities in
Solution: Multiplying each term by 4, we get \[\Rightarrow ~-20\text{ }\le \text{ }2\text{ }\text{ }-3x\text{ }\le \text{ }36\] Adding -2 each term, we get \[\Rightarrow ~-22\text{ }\le \text{...
Solve for x, the inequalities in |x – 1| ≤ 5, |x| ≥ 2
\[\left| x\text{ }\text{ }-1 \right|\le \text{ }5\] There are two cases, 1:-\[x\text{ }\text{ }-1\text{ }\le \text{ }5\] Adding 1 to LHS and RHS \[\begin{array}{*{35}{l}} \Rightarrow ~x\text{ }\le...
Solve for x, the inequalities in
Solution: \[\begin{array}{*{35}{l}} \Rightarrow ~5-\text{ }\text{ }\left| x \right|\text{ }\le \text{ }0\text{ }and\text{ }\left| x \right|\text{ }\text{ }-3\text{ }>\text{ }0\text{ }or\text{...
Solve for x, the inequalities in
Solution: Hence, \[\begin{array}{*{35}{l}} 1\text{ }\le \text{ }y\text{ }<\text{ }2 \\ \Rightarrow ~1\text{ }\le \text{ }\left| x-\text{ }\text{ }2 \right|\text{ }<\text{ }2 \\ \end{array}\]...
Solve for x, the inequalities in
SOLUTION: Multiplying each term by \[\begin{array}{*{35}{l}} \left( x\text{ }+\text{ }1 \right) \\ \Rightarrow ~4\text{ }\le \text{ }3\left( x\text{ }+\text{ }1 \right)\text{ }\le \text{ }6 \\...
If z and w are two complex numbers such that |zw| = 1 and arg (z) – arg (w) = π/2, then show that z̅w = – i.
Let z = \[\left| z \right|\text{ }(cos\text{ }{{\theta }_{1}}~+\text{ }I\text{ }sin\text{ }{{\theta }_{1}})\text{ }and\text{ }w\text{ }=\text{ }\left| w \right|\text{ }(cos\text{ }{{\theta...
Show that the magnetic field 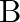 at a point in between the plates of a parallel plate capacitor during charging is
at a point in between the plates of a parallel plate capacitor during charging is 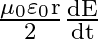
Let $I_{d}$ be the displacement current in the magnetic field region between two parallel plate capacitor plates. The magnetic field induction at a point between two capacitor plates at a...
Write the complex number in polar from.
SOLUTION:
Find the complex number satisfying the equation z + √2 |(z + 1)| + i = 0.
\[z\text{ }+\text{ }\surd 2\text{ }\left| \left( z\text{ }+\text{ }1 \right) \right|\text{ }+\text{ }i\text{ }=\text{ }0\text{ }\ldots \text{ }\left( 1 \right)\] Substituting\[z\text{ }=\text{...
Solve the system of equations Re (z2) = 0, |z| = 2.
\[\begin{array}{*{35}{l}} Re\text{ }({{z}^{2}})\text{ }=\text{ }0,\text{ }\left| z \right|\text{ }=\text{ }2 \\ Let\text{ }z\text{ }=\text{ }x\text{ }+\text{ }iy. \\ Then,\text{ }\left| z...
Poynting vectors 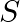 is defined as a vector whose magnitude is equal to the wave intensity and whose direction is along the direction of wave propagation. Mathematically, it is given by
is defined as a vector whose magnitude is equal to the wave intensity and whose direction is along the direction of wave propagation. Mathematically, it is given by 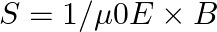 . Show that nature of
. Show that nature of 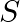 versus
versus 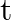 graph.
graph.
A variable frequency a.c source is connected to a capacitor. How will the displacement current change with a decrease in frequency?
Capacitive reaction is given as, $\mathrm{Xc}=1 / 2 \mathrm{mf} \mathrm{C}$ As the frequency decreases, $X_ c$ rises, and the conduction current becomes inversely proportional to $X_ c$.
If for complex numbers z1 and z2, arg (z1) – arg (z2) = 0, then show that |z1 – z2| = |z1| – |z2|.
Let \[{{z}_{1}}~=\text{ }\left| {{z}_{1}} \right|\text{ }\left( cos\text{ }{{\theta }_{1}}~+\text{ }I\text{ }sin\text{ }{{\theta }_{1}} \right)\text{ }and\text{ }{{z}_{2}}~=\text{ }\left| {{z}_{2}}...
The charge on a parallel plate capacitor varies as 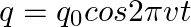 . The plates are very large and close together. Neglecting the edge effects, find the displacement current through the capacitor?
. The plates are very large and close together. Neglecting the edge effects, find the displacement current through the capacitor?
Displacement current through the capacitor is given as, $\mathrm{Id}=\mathrm{Ic}=\mathrm{dq} / \mathrm{dt}$ Given, $q=q_{0} \cos 2 \pi v t$ On putting the values, we get $\mathrm{Id}=\mathrm{Ic}=-2...
Why does a microwave oven heats up a food item containing water molecules most efficiently?
Because the microwave's frequency and the resonant frequency of the water molecules are the same, the microwave oven cooks a food item containing water molecules most efficiently.
Why is the orientation of the portable radio with respect to broadcasting station important?
As electromagnetic waves are plane polarised, the antenna must be parallel to the vibration of the fields of the wave. The orientation of the portable radio with respect to the transmitting station...
An EM wave of intensity I falls on a surface kept in vacuum and exerts radiation pressure 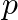 on it. Which of the following are true?
on it. Which of the following are true?
a) radiation pressure is 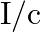 if the wave is totally absorbed
if the wave is totally absorbed
b) radiation pressure is 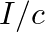 if the wave is totally reflected
if the wave is totally reflected
c) radiation pressure is 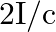 if the wave is totally reflected
if the wave is totally reflected
d) radiation pressure is in the range 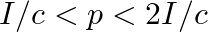 for real surface
for real surface
The correct options are: a) radiation pressure is $\mathrm{l} / \mathrm{c}$ if the wave is totally absorbed c) radiation pressure is $2 \mathrm{l} / \mathrm{c}$ if the wave is totally reflected d)...
A charged particle oscillates about its mean equilibrium position with a frequency of 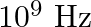 . The electromagnetic waves produced:
. The electromagnetic waves produced:
a) will have a frequency of 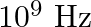
b) will have a frequency of 
c) will have a wavelength of 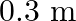
d) fall in the region of radiowaves
The correct options are: a) will have a frequency of $10^{9} \mathrm{~Hz}$ c) will have a wavelength of $0.3 \mathrm{~m}$ d) fall in the region of radiowaves
If |z1| = |z2| = ….. = |zn| = 1, then show that |z1 + z2 + z3 + …. + zn| = | 1/z1 + 1/z2 + 1/z3 + … + 1/zn|
\[\begin{array}{*{35}{l}} \left| {{z}_{1}} \right|\text{ }=\text{ }\left| {{z}_{2}} \right|\text{ }=\text{ }\ldots \text{ }=\text{ }\left| {{z}_{n}} \right|\text{ }=\text{ }1 \\ \Rightarrow...
If z1, z2 and z3, z4 are two pairs of conjugate complex numbers, then find arg(z1/z4) + arg(z2/z3).
z1 and z2 are conjugate complex numbers. The negative side of the real axis \[\begin{array}{*{35}{l}} =\text{ }{{r}_{1}}~\left( cos\text{ }{{\theta }_{1}}~\text{ }-i\text{ }sin\text{ }{{\theta...
If |z1| = 1 (z1 ≠ –1) and z2 = (z1 – 1) / (z + 1), then show that the real part of z2 is zero.
Let z1 = x + iy Therefore, the real part of z2 is zero.
z1 and z2 are two complex numbers such that |z1| = |z2| and arg (z1) + arg (z2) = π, then show that z1 = – z̅2.
According to the question, Let \[{{z}_{1}}~=\text{ }|{{z}_{1}}|\text{ }(cos\text{ }{{\theta }_{1}}~+\text{ }I\text{ }sin\text{ }{{\theta }_{1}})\text{ }and\text{ }{{z}_{2}}~=\text{...
If Y = {1, 2, 3,…, 10}, and a represents any element of Y, write the following sets, containing all the elements satisfying the given conditions. (i) a is less than 6 and a ∈ Y
Solution: (i) As per the question, $Y=\{1,2,3, \ldots, 10\}$ in which a represents any element of $Y$ $Y=\{1,2,3, \ldots, 10\}$ $a$ is less than $6 \Rightarrow 1,2,3,4,5$ $1,2,3,4,5$ satisfy the...
If Y = {1, 2, 3,…, 10}, and a represents any element of Y, write the following sets, containing all the elements satisfying the given conditions. (i) a ∈ Y but a2∉ Y (ii) a + 1 = 6, a ∈ Y
Solution: (i) As per the question, $Y=\{1,2,3, \ldots, 10\}$ in which a represents any element of $Y$ $\begin{array}{l} Y=\{1,2,3, \ldots, 10\} \\ 1^{2}=1,2^{2}=4,3^{2}=9 \end{array}$ $1,4,9 \in Y...
If (z – 1)/(z + 1) is a purely imaginary number (z ≠ –1), then find the value of |z|.
\[Let\text{ }z\text{ }=\text{ }x\text{ }+\text{ }iy\] Now, \[\begin{array}{*{35}{l}} \Rightarrow ~{{x}^{2}}~\text{ }-1\text{ }+\text{ }{{y}^{2}}~=\text{ }0 \\ \Rightarrow ~{{x}^{2}}~+\text{...
Show that |(z – 2) / (z – 3)| = 2 represents a circle. Find its centre and radius.
\[\begin{array}{*{35}{l}} arg(\left| \left( z\text{ }\text{ }-2 \right)\text{ }/\text{ }\left( z-\text{ }\text{ }3 \right) \right|\text{ })=\text{ }2 \\ Substituting\text{ }z\text{ }=\text{...
If X = {1, 2, 3}, if n represents any member of X, write the following sets containing all numbers represented by: (i) n/2 (ii) n – 1
Solution: (i) As per the question, $X=\{1,2,3\}$ in which $n$ represents any member of $X$ $X=\{1,2,3\}$ (ii) As per the question, $X=\{1,2,3\}$ in which $n$ represents any member of $X$...
If arg (z – 1) = arg (z + 3i), then find x – 1 : y. where z = x + iy
Let \[z\text{ }=\text{ }x\text{ }+\text{ }iy\] Given that, \[arg\text{ }\left( z-\text{ }\text{ }1 \right)\text{ }=\text{ }arg\text{ }\left( z\text{ }+\text{ }3i \right)\] \[\Rightarrow ~arg\text{...
If X = {1, 2, 3}, if n represents any member of X, write the following sets containing all numbers represented by: (i) 4n (ii) n + 6
Solution: (i) As per the question, $X=\{1,2,3\}$ in which $n$ represents any member of $X$ $\begin{array}{l} X=\{1,2,3\} \\ \{4 n \mid n \in x\} \\ =\{4 \times 1,4 \times 2,4 \times 3\} \\...
If |z + 1| = z + 2 (1 + i), then find z.
\[\left| z\text{ }+\text{ }1 \right|\text{ }=\text{ }z\text{ }+\text{ }2\text{ }\left( 1\text{ }+\text{ }i \right)\] Substituting z = x + iy, we get, \[\Rightarrow ~\left| x\text{ }+\text{ }iy\text{...
Given that N = {1, 2, 3,…, 100}. Then write (i) the subset of N whose elements are even numbers. (ii) the subset of N whose element are perfect square numbers.
Solution: It is known that, A set 'A' is a subset of a set 'B', if 'A' is "contained" inside 'B'. As a result, all elements of 'A' are also elements of 'B'. (i) As per the question, N$=\{1,2,3,...
Solve that equation |z| = z + 1 + 2i.
As per the inquiry, We have, \[\left| z \right|\text{ }=\text{ }z\text{ }+\text{ }1\text{ }+\text{ }2i\] Subbing z = x + iy, we get, \[\begin{array}{*{35}{l}} \Rightarrow \left| x\text{ }+\text{ }iy...
If A and B are subsets of the universal set U, then show that (i) (A ∩ B) ⊂ A
Solution: (i) As per the question, Two subsets are A and B We need to prove: $(A \cap B) \subset A$ Proof: Let's say $x \in A \cap B$ $\Rightarrow x \in A$ and $x \in B$ $\Rightarrow \mathrm{X} \in...
If A and B are subsets of the universal set U, then show that (i) A ⊂ A ∪ B (ii) A ⊂ B ⇔ A ∪ B = B
Solution: (i) As per the question, $A$ and $B$ are two subsets We need to prove: $A \subset A \cup B$ Proof: Let's say $x \in A$ $\Rightarrow \mathrm{x} \in \mathrm{A}$ or $\mathrm{x} \in...
Show that the complex number z, satisfying the condition arg ((z-1)/(z+1)) = π/4 lies on a circle.
Let \[z\text{ }=\text{ }x\text{ }+\text{ }iy\] arg \[\left( \left( z-1 \right)/\left( z+1 \right) \right)\text{ }=\text{ }\pi /4\] \[\Rightarrow ~arg\text{ }\left( z\text{ }\text{ }1 \right)\text{...
If the real part of ( z̅ + 2)/ ( z̅ – 1) is 4, then show that the locus of the point representing z in the complex plane is a circle.
Let z = x + iy Now, \[\Rightarrow ~{{x}^{2}}~+\text{ }x\text{ }\text{ }-2\text{ }+\text{ }{{y}^{2}}~=\text{ }4\text{ }\left( {{x}^{2}}~\text{ }-2x\text{ }+\text{ }1\text{ }+\text{ }{{y}^{2}}...
If z = x + iy, then show that zz̅ + 2(z + z̅) + b = 0 where bϵR, representing z in the complex plane is a circle.
\[z\text{ }=\text{ }x\text{ }+\text{ }iy\] ⇒ z̅ = x – iy Now, we also have, z z̅ + 2 (z + z̅) + b = 0 \[\Rightarrow ~\left( x\text{ }+\text{ }iy \right)\text{ }\left( x\text{ }\text{ }iy...
If (1 + i)z = (1 – i) z̅, then show that z = i z̅.
SOLUTION: = -iz̅ Hence proved.
If a = cos θ + i sin θ, find the value of
SOLUTION: a = cos θ + i sin θ
IF
then find (a, b). Solution: = (i4)25 = 1 Hence, (a, b) = (1, 0)
If (FIG 1) then find the value of x + y.
FIG 1: SOLUTION: We have,
If (fig 1), then find (x, y).
fig 1: SOLUTION: We have,
Evaluate
where n ϵ N. SOLUTION: WE have,
For a positive integer n, find the value of (1 – i)^n (1 – 1/i)^n.
A/Q = (1 – i)n (1 + i)n = (1 – i2)n = 2n Therefore, (1 – i)n (1 – 1/i)n = 2n
1 + 2 + 2^2 + … 2^n = 2n+1 – 1 for all natural numbers n.
Let P(n):\[1\text{ }+\text{ }2\text{ }+\text{ }{{2}^{2}}~+\text{ }\ldots \text{ }+\text{ }{{2}^{n}}~=\text{ }{{2}^{n}}{{~}^{+1}}~\text{ }1\] , for all natural numbers n \[P\left( 1 \right):\text{...
Prove √n < 1/√1 + 1/√2 + … 1/√n, for all natural numbers n ≥ 2.
As per the inquiry, \[\Rightarrow P\left( k+1 \right)\] is valid when P(k) is valid. Along these lines, by Mathematical Induction, \[\surd n\text{ }<\text{ }1/\surd 1\text{ }+\text{ }1/\surd...
Given L = {1, 2, 3, 4}, M = {3, 4, 5, 6} and N = {1, 3, 5}. Verify that L–(M∪N) = (L–M)∩(L–N).
Solution: As per the question, $\mathrm{L}=\{1,2,3,4\}, \mathrm{M}=\{3,4,5,6\}$ and $\mathrm{N}=\{1,3,5\}$ We need to verify: $\begin{array}{l} L-(M \cup N)=(L-M) \cap(L-N) \\ M=\{3,4,5,6\},...
Prove n^2 < 2n for all natural numbers n ≥ 5.
As indicated by the inquiry, \[P\left( n \right)\text{ }is\text{ }n\hat{\ }^2\text{ }<\text{ }2n\text{ }for\text{ }n\ge 5\] Let \[P\left( k \right)\text{ }=\text{ }k^2\text{ }<\text{ }2k\] be...
State which of the following statements are true and which are false. Justify your answer. (i) 3 ∉ {x | x4 – 5×3 + 2×2 – 112x + 6 = 0} (ii) 496 ∉ {y | the sum of all the positive factors of y is 2y}.
Solution: (i) The statement is true As per the question, $\begin{array}{l} 3 \notin\left\{x \mid x^{4}-5 x^{3}+2 x^{2}-112 x+6=0\right\} \\ x^{4}-5 x^{3}+2 x^{2}-112 x+6=0 \end{array}$ When we put...
Prove n(n^2 + 5) is divisible by 6, for each natural number n.
As per the inquiry, \[P\left( n \right)\text{ }=\text{ }n\left( n^2\text{ }+\text{ }5 \right)\] is distinguishable by 6. Along these lines, subbing various qualities for n, we get, \[P\left( 0...
Prove For any natural number n, x^n – y^n is divisible by x – y, where x integers with x ≠ y.
As indicated by the inquiry, \[P\left( n \right)\text{ }=\text{ }xn\text{ }\text{ }yn\] is detachable by \[x\text{ }\text{ }y,\text{ }x\] whole numbers with\[x\text{ }\ne \text{ }y\] . In this way,...
Prove 3^2n – 1 is divisible by 8, for all natural numbers n.
As indicated by the inquiry, \[P\left( n \right)\text{ }=\text{ }32n\text{ }\text{ }1\] is distinct by 8. Along these lines, subbing various qualities for n, we get, \[P\left( 0 \right)\text{...
A plane electromagnetic wave propagating along 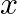 -direction can have the following pairs of
-direction can have the following pairs of  and
and 
a) 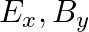
b) 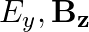
c) 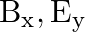
d) 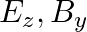
The correct options are: b) $E_y, \mathbf{B_ z}$ d) $E_z, B_y$
Prove 2^3n – 1 is divisible by7, for all natural numbers n.
As indicated by the inquiry, \[P\left( n \right)\text{ }=\text{ }23n\text{ }\text{ }1\] is detachable by 7. In this way, subbing various qualities for n, we get, \[P\left( 0 \right)\text{ }=\text{...
An electromagnetic wave travelling along the  -axis is given as:
-axis is given as: 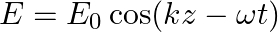 . Choose the correct options from the following a) the associated magnetic field is given as
. Choose the correct options from the following a) the associated magnetic field is given as 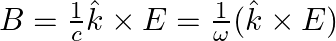
b) the electromagnetic field can be written in terms of the associated magnetic field as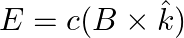
c)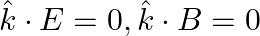
d)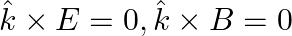
a) the associated magnetic field is given as $B=\frac{1}{c} \hat{k} \times E=\frac{1}{\omega}(\hat{k} \times E)$ b) the electromagnetic field can be written in terms of the associated magnetic field...
Write the following sets in the roaster form: (i) 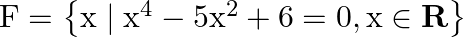
Solution: (i) As per the question, $F=\left\{x \mid x^{4}-5 x^{2}+6=0, x \in R\right\}$ Roster form, $\begin{array}{l} x^{4}-5 x^{2}+6=0 \\ \Rightarrow x^{4}-3 x^{2}-2 x^{2}+6=0 \\ \Rightarrow...
Write the following sets in the roaster form: (i) 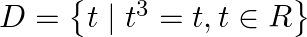 (ii)
(ii) 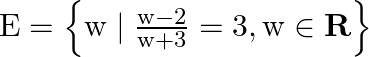
Solution: (i) As per the question, $D=\left\{t \mid t^{3}=t, t \in R\right\}$ Roster form, $\begin{array}{l} t^{3}=t \\ \Rightarrow t^{3}-t=0 \\ \Rightarrow t\left(t^{2}-1\right)=0 \\ \Rightarrow...
Write the following sets in the roaster form: (i) C = {x | x is a positive factor of a prime number p}
Solution: (i) As per the question, $C=\{x \mid x$ is a positive factor of a prime number $p\}$ Roster form, $p=1$ and $p$ itself are the only possible positive factors of a prime number. Hence,...
Write the following sets in the roaster form: (i) A = {x : x ∈ R, 2x + 11 = 15} (ii) B = {x | x2 = x, x ∈ R}
Solution: (i) As per the question, $A=\{x: x \in R, 2 x+11=15\}$ Roster form, $\begin{array}{l} 2 x+11=15 \\ \Rightarrow 2 x=15-11 \\ \Rightarrow 2 x=4 \\ \Rightarrow x=2 \end{array}$ As a result,...
The ratio of contributions made by the electric field and magnetic field components to the intensity of an EM wave is
a) 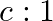
b) 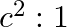
c) 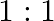
d) 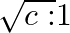
c) $1: 1$
The electric field intensity produced by the radiations coming from 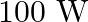 bulb at a
bulb at a 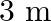 distance is E. The electric field intensity produced by the radiations coming from
distance is E. The electric field intensity produced by the radiations coming from 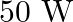 bulb at the same distance is
bulb at the same distance is
a) 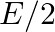
b) 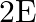
c) 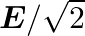
d) 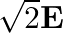
c) $\mathrm{E} / \sqrt{2}$
Light with an energy flux of 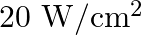 falls on a non-reflecting surface at normal incidence. If the surface has an area of
falls on a non-reflecting surface at normal incidence. If the surface has an area of 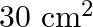 , the total momentum delivered during 30 minutes is
, the total momentum delivered during 30 minutes is
a) 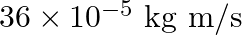
b) 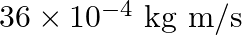
c) 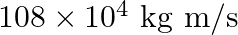
d) 
b) $36 \times 10^{-4} \mathrm{~kg} \mathrm{~m} / \mathrm{s}$
A linearly polarized electromagnetic wave given as 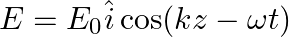 is incident normally on a perfectly reflecting infinite wall at
is incident normally on a perfectly reflecting infinite wall at  .
.
Assuming that the material of the wall is optically inactive, the reflected wave will be given as
a)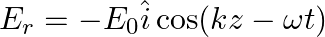
b)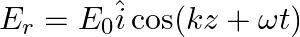
c)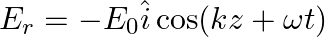
d)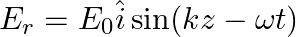
b)$E_{r}=E_{0} \hat{i} \cos (k z+\omega t)$
In the LCR circuit the ac driving voltage is v = vm sin ωt.
(i) Write down the equation of motion for q (t).
(ii) At 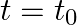 , the voltage source stops and R is short-circuited. Now write down how much energy is stored in each of L and C.
, the voltage source stops and R is short-circuited. Now write down how much energy is stored in each of L and C.
i) The equation for charge motion variation with respect to time is as follows: $\mathrm{L} d^{2} q(\mathrm{t}) / \mathrm{dt}+\mathrm{R} \mathrm{dq}(\mathrm{t}) / \mathrm{dt}+\mathrm{q}(\mathrm{t})...
Give an example of a statement P(n) which is true for all n. Justify your answer.
As indicated by the inquiry, P(n) which is valid for all n. Let P(n) be, ⇒ P(k) is valid for all k. In this manner, P(n) is valid for all n.
Give an example of a statement P(n) which is true for all n ≥ 4 but P(1), P(2) and P(3) are not true. Justify your answer.
As indicated by the inquiry, P(n) which is valid for all n ≥ 4 however P(1), P(2) and P(3) are false Let \[P\left( n \right)\text{ }be\text{ }2n\text{ }<\text{ }n!\] Thus, the instances of the...
For an LCR circuit driven at frequency ω, the equation reads L di/dt + Ri + q/C = vi = vm sin ꞷ t
(i) Multiply the equation by i and simplify where possible.
(ii) Interpret each term physically.
Given equation is, $L d i / d t+R i+q / C=v i=v m \sin \omega t$ i) On multiplying the above equation with I, we get, $\mathrm{d}\left(1 / 2 \mathrm{Li}^{2}\right) / \mathrm{dt}+1 / 2 \mathrm{C}...
1MW power is to be delivered from a power station to a town 10 km away. One uses a pair of Cu wires of radius 0.5 cm for this purpose. Calculate the fraction of ohmic losses to power transmitted if
(i) power is transmitted at 220V. Comment on the feasibility of doing this.
(ii) a step-up transformer is used to boost the voltage to 11000 V, power transmitted, then a step-down transformer is used to bring the voltage to 220 V.
i) It is given that the power station is $10 \mathrm{~km}$ away from the town Length of the $\mathrm{Cu}$ wire is given as $\mathrm{L}=20 \mathrm{~km}=20000 \mathrm{~m}$ Resistance of $\mathrm{Cu}$...
Explain why the reactance offered by an inductor increases with increasing frequency of an alternating voltage.
The reactance offered by an inductor increases with the increasing frequency of an alternating voltage because the induced emf is proportional to the rate of change of current.
A 60 W load is connected to the secondary of a transformer whose primary draws line voltage. If a current of 0.54 A flows in the load, what is the current in the primary coil? Comment on the type of transformer being used.
$P_{s}$ is given as $60W$ $I_{s}$ is given as $0.54A$ Primary voltage is given as $220V$ $V_{s}=60/0.54=110V$ The transformer is a step-down transformer because the secondary voltage is lower than...
A device ‘X’ is connected to an a.c source. The variation of voltage, current and power in one complete cycle is shown in the figure.
Identify the device ‘X’.
Solution: X could be an inductor, a capacitor, or a hybrid of the two.
How does the sign of the phase angle φ, by which the supply voltage leads the current in an LCR series circuit, change as the supply frequency is gradually increased from very low to very high values.
$tan \phi = \frac{X_{l}-X_{c}}{R}$ $X_{l}<X_{c}$ At resonate frequency we have, $X_{l}=X_{c}$ $tan \phi = 0$
In series LCR circuit, the plot of I max vs ω is shown in the figure. Find the bandwidth and mark in the figure.
Frequency 1 is 0.8 rad/s Frequency 2 is 1.2 rad/s The bandwidth is 0.4 rad/s
Study the circuits (a) and (b) shown in the figure and answer the following questions.
(a) Under which conditions would the rms currents in the two circuits be the same? (b) Can the rms current in the circuit (b) be larger than that in (a)? Solution: a) $(I_{rms})a=(I_{rms})b$...
If an LC circuit is considered analogous to a harmonically oscillating spring block system, which energy of the LC circuit would be analogous to potential energy and which one analogous to kinetic energy?
The magnetic energy of the LC circuit is equivalent to kinetic energy, while electrostatic energy due to a capacitor's charge change is analogous to potential energy.
When an AC voltage of 220 V is applied to the capacitor C
(a) the maximum voltage between plates is 220 V.
(b) the current is in phase with the applied voltage.
(c) the charge on the plates is in phase with the applied voltage.
(d) power delivered to the capacitor is zero.
The correct options are: (c) the charge on the plates is in phase with the applied voltage. (d) power delivered to the capacitor is zero.
Electrical energy is transmitted over large distances at high alternating voltages. Which of the following statements is (are) correct?
(a) For a given power level, there is a lower current.(b) Lower current implies less power loss.
(c) Transmission lines can be made thinner.
(d) It is easy to reduce the voltage at the receiving end using step-down transformers.
The correct options are: (a) For a given power level, there is a lower current. (b) Lower current implies less power loss. (d) It is easy to reduce the voltage at the receiving end using step-down...
As the frequency of an ac circuit increases, the current first increases and then decreases. What combination of circuit elements is most likely to comprise the circuit?
(a) Inductor and capacitor.
(b) Resistor and inductor.
(c) Resistor and capacitor.
(d) Resistor, inductor and capacitor.
The correct options are: (a) Inductor and capacitor. (d) Resistor, inductor and capacitor.
An inductor of reactance 1 Ω and a resistor of 2 Ω are connected in series to the terminals of a 6 V (rms) a.c. source. The power dissipated in the circuit is
(a) 8 W.
(b) 12 W.
(c) 14.4 W.
(d) 18 W.
(c) 14.4 W.
To reduce the resonant frequency in an LCR series circuit with a generator
(a) the generator frequency should be reduced.
(b) another capacitor should be added in parallel to the first.
(c) the iron core of the inductor should be removed.
(d) dielectric in the capacitor should be removed.
(b) another capacitor should be added in parallel to the first
When a voltage measuring device is connected to AC mains, the meter shows the steady input voltage of 220V. This means
(a) input voltage cannot be AC voltage, but a DC voltage.
(b) maximum input voltage is 220V.
(c) the meter reads not v but 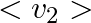 and is calibrated to read
and is calibrated to read 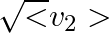 .
.
(d) the pointer of the meter is stuck by some mechanical defect.
(c) the meter reads not v but $<v_{2}>$ and is calibrated to read $\sqrt <v_{2}>$
An alternating current generator has an internal resistance 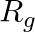 and an internal reactance
and an internal reactance 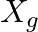 . It is used to supply power to a passive load consisting of a resistance
. It is used to supply power to a passive load consisting of a resistance 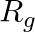 and a reactance
and a reactance 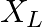 . For maximum power to be delivered from the generator to the load, the value of
. For maximum power to be delivered from the generator to the load, the value of 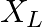 is equal to
is equal to
(a) zero.
(b) 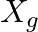 .
.
(c) 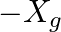 .
.
(d) 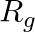 .
.
(c) $-X_{g}$.
What are the advantages of the null-point method in a Wheatstone bridge? What additional measurements would be required to calculate R unknown by any other?
Answer: It is advantageous to use a null-point in the Wheatstone bridge because it prevents any effect on the resistance of the galvanometer caused by the balance point. Kirchhoff's rule is used to...
The relaxation time τ is nearly independent of applied E field whereas it changes significantly with temperature T. First fact is responsible for Ohm’s law whereas the second fact leads to a variation of ρ with temperature. Elaborate why?
Answer: The relaxation time (average time between successive collisions) decreases as the drift velocity increases, increasing the...
Is the momentum conserved when charge crosses a junction in an electric circuit? Why or why not?
Answer: When a charge traverses a junction in an electric circuit, the momentum of the charge does not conserve. This is due to the fact that the drift velocity is directly proportional to the...
Earth’s orbit is an ellipse with eccentricity 0.0167. Thus, the earth’s distance from the sun and speed as it moves around the sun varies from day to day. This means that the length of the solar day is not constant throughout the year. Assume that earth’s spin axis is normal to its orbital plane and find out the length of the shortest and the longest day. A day should be taken from noon to noon. Does this explain the variation of the length of the day during the year?
Solution: Velocity of the earth at perigee is given as $v_{p}$ Velocity of the earth at apogee is given as $v_{a}$ Angular velocity of the earth at perihelion is given as $\omega_{p}$ Angular...
Six-point masses of mass m each are at the vertices of a regular hexagon of side l. Calculate the force on any of the masses.
$AE = AG + EG$ $AG + AG = 2AG$ $= 2l cos 30^{o}$ $AE = AC = \sqrt3l$ $AD = 2l$ As a result, $\frac{Gm^{2}}{l^{2}}$ is the force on A due to B along B to A $\frac{Gm^{2}}{3l^{2}}$ is the force on A...
A star like the sun has several bodies moving around it at different distances. Consider that all of them are moving in circular orbits. Let r be the distance of the body from the centre of the star and let its linear velocity be v, angular velocity ω, kinetic energy K, gravitational potential energy U, total energy E, and angular momentum l. As the radius r of the orbit increases, determine which of the above quantities increase and which ones decrease.
When a body moves around a star in equilibrium, the gravitational attraction produces a centripetal force. Consider a body of mass $m$ revolving in a circular path of radius $r$ around the star S of...
A mass m is placed at P a distance h along the normal through the centre O of a thin circular ring of mass M and radius r. If the mass is removed further away such that OP becomes 2h, by what factor the force of gravitation will decrease, if h = r?
Solution: Let the radius of the ring be r Let the mass of the ring be m When small element dM is considered as the mass, the gravitation force becomes, $dF=\frac{G(dM)m}{x^{2}}$ where...
Shown are several curves. Explain with reason, which ones amongst them can be possible trajectories traced by a projectile
Solution: Amongst the given figures, (c) show the focus of trajectory.
Show the nature of the following graph for a satellite orbiting the earth.
a) KE vs orbital radius R
b) PE vs orbital radius R
a) $\mathrm{K}=1 / 2 \mathrm{mv}^{2}=(1 / 2 \mathrm{~m})(\mathrm{GM} / \mathrm{R})$ b) PE of satellite is $U = -GMm/R = -2K$
Mean solar day is the time interval between two successive noon when the sun passes through zenith point. The sidereal day is the time interval between two successive transits of a distant star through the zenith point. By drawing the appropriate diagram showing earth’s spin and orbital motion, show that mean solar day is four minutes longer than the sidereal day. In other words, distant stars would rise 4 minutes early every successive day.
The polar axis of the earth and its movement are E and E’ respectively. Translational motion is P’ After every 24 hours, earth's orbit is approximately advanced by $1^{o}$ As a result, time taken...
Out of aphelion and perihelion, where is the speed of the earth more and why?
According to Kepler’s second law, a real velocity is constant and is given as: $r_{p}\times v_{p}=r_{A}\times v_{A}$ $\frac{r_{A}}{r_{p}}=\frac{v_{p}}{v_{A}}$ $r_{A}>r_{p}$ and...
The gravitational force between a hollow spherical shell and a point mass is F. Show the nature of F vs r graph where r is the distance of the point from the centre of the hollow spherical shell of uniform density.
R is the spherical shell's radius, while r is the distance between m and M. The point's mass is m, while the hollow spherical shell's mass is M. Now, $F=\frac{GMm}{r^{2}}$ When F = 0,...
An astronaut inside a small spaceship orbiting around the earth cannot detect gravity. If the space station orbiting around the earth has a large size, can he hope to detect gravity?
The astronaut will experience variation when the size of the space station orbiting the earth is large, and this is due to acceleration due to gravity.
Is it possible for a body to have inertia but no weight?
Yes. It is possible for a body to have inertia but no weight as inertia is associated with the mass of the body. Satellite revolving around the earth is an example of a body with inertia and has no...
What is the direction of areal velocity of the earth around the sun?
The direction of the earth's areal velocity around the sun is determined by the product of r and v.
Give one example each of central force and non-central force.
Example of Central force: Electrostatic force acting on the point charge Example of Non-central force: Nuclear force between the atoms
Molecules in the air in the atmosphere are attracted by the gravitational force of the earth. Explain why all of them do not fall into the earth just like an apple falling from a tree.
The gravitational force of the earth attracts molecules in the atmosphere, yet they do not descend into the earth since they are in random motion, whereas an apple moves downhill.
There have been suggestions that the value of the gravitational constant G becomes smaller when considered over a very large time period in the future. If that happens for our earth,
a) nothing will change
b) we will become hotter after billions of years
c) we will be going around but not strictly in closed orbits
d) after a sufficiently long time we will leave the solar system
The correct options are c) we will be going around but not strictly in closed orbits d) after a sufficiently long time we will leave the solar system
If the mass of sun were ten times smaller and gravitational constant G were ten times larger in magnitudes
a) walking on ground would become more difficult
b) the acceleration due to gravity on earth will not change
c) raindrops will fall much faster
d) aeroplanes will have to travel much faster
The correct options are a) walking on ground would become more difficult c) raindrops will fall much faster d) aeroplanes will have to travel much faster
Particles of masses 2M, m and M are respectively at points A, B, and C with AB = 1/2 (BC). M is much-much smaller than M and at time t = 0, they are all at rest. At subsequent times before any collision takes place
a) m will remain at rest b) m will move towards M c) m will move towards 2M d) m will have oscillatory motion Solution: The correct option is c) m will move towards 2M
In our solar system, the inter-planetary region has chunks of matter called asteroids. They
a) will not move around the sun since they have very small masses compared to the sun
b) will move in an irregular way because of their small masses and will drift away outer space
c) will move around the sun in closed orbits but not obey Kepler’s laws
d) will move in orbits like planets and obey Kepler’s laws
The correct option is d) will move in orbits like planets and obey Kepler’s laws
Satellites orbiting the earth have a finite life and sometimes debris of satellites fall to the earth. This is because
a) the solar cells and batteries in satellites run out
b) the laws of gravitation predict a trajectory spiralling inwards
c) of viscous forces causing the speed of the satellite and hence height to gradually decrease
d) of collisions with other satellites
The correct option is c) of viscous forces causing the speed of the satellite and hence height to gradually decrease
As observed from earth, the sun appears to move in an approximately circular orbit. For the motion of another planet like mercury as observed from earth, this would
a) be similarly true
b) not be true because the force between earth and mercury is not inverse square law
c) not be true because the major gravitational force on mercury is due to sun
d) not be true because mercury is influenced by forces other than gravitational forces
The correct option is c) not be true because the major gravitational force on mercury is due to sun
The earth is an approximate sphere. If the interior contained matter which is not of the same density everywhere, then on the surface of the earth, the acceleration due to gravity
a) will be directed towards the centre but not the same everywhere
b) will have the same value everywhere but not directed towards the centre
c) will be same everywhere in magnitude directed towards the centre
d) cannot be zero at any point
The correct option is d) cannot be zero at any point
Which of the following are the correct reasons for the anomalous behaviour of lithium? (i) The exceptionally small size of its atom (ii) Its high polarising power (iii) It has a high degree of hydration (iv) Exceptionally low ionisation enthalpy
Answer: Option i) & ii) Lithium exhibits a high ionization enthalpy in addition to a high degree of hydration. This is owing to the fact that it is so little.
Choose the correct statements from the following. (i) Beryllium is not readily attacked by acids because of the presence of an oxide film on the surface of the metal. (ii) Beryllium sulphate is readily soluble in water as the greater hydration enthalpy of Be2+ overcomes the lattice enthalpy factor. (iii) Beryllium exhibits coordination number more than four. (iv) Beryllium oxide is purely acidic.
Answer: Option i) & ii) Be mimics Al (diagonal relationship), and together they create a protective film of oxide that is resistant to acid assault. Because of the high hydration enthalpy of...
Identify the correct formula of halides of alkaline earth metals from the following. (i) BaCl2.2H2O (ii) BaCl2.4H2O (iii) CaCl2.6H2O (iv) SrCl2.4H2O
Answer: option i) & iii) The tendency to generate halide hydrates decreases gradually as one moves down the chemical group. The hydrates are MgCl2.6H2O, CaCl2.6H2O, SrCl2.6H2O, and BaCl2.2H2O.
When Zeolite, which is hydrated sodium aluminium silicate is treated with hard water, the sodium ions are exchanged with which of the following ion(s)? (i) 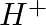 ions (ii)
ions (ii) 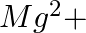 ions (iii)
ions (iii) 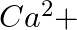 ions (iv)
ions (iv) 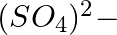 ions
ions
Answer: Option ii) & iii) Because of this, when zeolite, which is sodium aluminium silicate, reacts with hard water, the sodium ion of zeolite is swapped for calcium and magnesium ions.
Which of the following compounds are readily soluble in water? (i) BeSO4 (ii) MgSO4 (iii) BaSO4 (iv) SrSO4
Answer: Option i) & ii) Because their hydration enthalpies are higher than their lattice enthalpies, BeSO4 and MgSO4 are extremely soluble in water. CaSO4, SrSO4, and BaSO4 have low hydration...
Several sodium compounds find use in industries. Which of the following compounds are used for textile industry? (i) Na2CO3 (ii) NaHCO3 (iii) NaOH (iv) NaCl
Answer: Option i) & iii) Both the chemicals are widely used in industries for preparation of other compounds in large scale.
Metallic elements are described by their standard electrode potential, fusion enthalpy, atomic size, etc. The alkali metals are characterised by which of the following properties? (i) High boiling point (ii) High negative standard electrode potential (iii) High density (iv) Large atomic size
Answer: Option ii) & iv) Periods begin with alkali metals. For their period, alkali metals have the biggest atomic radius. They have low density due to their huge size and low bulk. Alkali...
Dehydration of hydrates of halides of calcium, barium and strontium i.e., CaCl26H2O, BaCl2.2H2O, SrCl2.2H2O, can be achieved by heating. These become wet on keeping in air. Which of the following statements is correct about these halides? (i) act as dehydrating agent (ii) can absorb moisture from the air (iii) The tendency to form hydrate decreases from calcium to barium (iv) All of the above
Answer: Option iv) Because they are hygroscopic in nature, the calcium, barium, and strontium halides operate as a dehydrating agent in the body. They are capable of absorbing moisture....
A chemical A is used for the preparation of washing soda to recover ammonia. When CO2 is bubbled through an aqueous solution of A, the solution turns milky. It is used in whitewashing due to disinfectant nature. What is the the chemical formula of A? (i) Ca (HCO3)2 (ii) Cao (iii) Ca(OH)2 (iv) CaCO3
Answer: option iii) Ca(OH)2 is the chemical A that is used in the manufacturing of washing soda Na2CO3 in order to recover ammonia. It is a reaction that occurs during the Solvay process, which is...
A substance which gives brick red flame and breaks down on heating to give oxygen and a brown gas is (i) Magnesium nitrate (ii) Calcium nitrate (iii) Barium nitrate (iv) Strontium nitrate
Answer: Option ii) The alkali metal compounds and alkaline earth metal compounds give the flame its colour. In a flame, calcium turns brick red, strontium turns crimson red, barium turns apple...
The formula of soda ash is (i) Na2CO3.10H2O (ii) Na2CO3.2H2O (iii) Na2CO3.H2O (iv) Na2CO3
Answer: Option iv) The formula for soda ash is $Na_2CO_3$ that is sodium carbonate.
Which of the following elements does not form hydride by direct heating with dihydrogen? (i) Be (ii) Mg (iii) Sr (iv) Ba
Answer: Option i) When heated, all of the elements, with the exception of beryllium, mix with hydrogen to create their hydrides.
Suspension of slaked lime in water is known as (i) lime water (ii) quick lime (iii) milk of lime (iv) an aqueous solution of slaked lime
Answer: Option iii) Calcium hydroxide dissociates in an aqueous solution, releasing calcium cations and hydroxide anions. Calcium oxide is the chemical compound known as quicklime. Milk of lime is a...
Dead burnt plaster is (i) CaSO4 (ii) CaSO4.1/2 H2O (iii) CaSO4.H2O (iv) CaSO4.2H2O
Answer: Option i) When plaster of Paris is heated to 200°C, it transforms into anhydrous calcium sulphate, often known as dead plaster, which lacks setting properties since it absorbs water at a...
By adding gypsum to cement (i) setting time of cement becomes less. (ii) setting time of cement increases. (iii) colour of cement becomes light. (iv) the shining surface is obtained.
Answer: Option ii) The addition of gypsum serves only to slow down the setting process of the cement, allowing it to solidify to a sufficiently hard state before use.
When sodium is dissolved in liquid ammonia, a solution of deep blue colour is obtained. The colour of the solution is due to (i) ammoniated electron (ii) sodium ion (iii) sodium amide (iv) ammoniated sodium ion
Answer: option i) The concentration of these solvated ions in the sodium in liquid ammonia solution changes the color to copper. So the ammoniated electron is responsible for the solution's color.
In the synthesis of sodium carbonate, the recovery of ammonia is done by treating NH4Cl with Ca(OH)2. The by-product obtained in this process is (i) CaCl2 (ii) NaCl (iii) NaOH (iv) NaHCO3
Answer: Option i) Sodium carbonate is synthesised by Solvary ammonia soda process.
Amphoteric hydroxides react with both alkalies and acids. Which of the following Group 2 metal hydroxides is soluble in sodium hydroxide? (i) Be(OH)2 (ii) Mg(OH)2 (iii) Ca(OH)2 (iv) Ba(OH)2
Answer: option i) The solubility of hydroxides of alkaline earth metals in water increases as the alkaline earth metals move from Be to Ba. Both Be(OH)2 and Mg(OH)2 are nearly insoluble in water....
The solubility of metal halides depends on their nature, lattice enthalpy and hydration enthalpy of the individual ions. Amongst fluorides of alkali metals, the lowest solubility of LiF in water is due to (i) Ionic nature of lithium fluoride (ii) High lattice enthalpy (iii) High hydration enthalpy for lithium-ion. (iv) Low ionisation enthalpy of the lithium atom
Answer: Option ii) Lattice and hydration enthalpies encourage the dissolving of alkali metal halides in water. Fluorides are soluble in this order: LiF, NaF, KF, RbF, CsF. The high lattice energy of...
Some of the Group 2 metal halides are covalent and soluble in organic solvents. Among the following metal halides, the one which is soluble in ethanol is (i) BeCl2 (ii) MgCl2 (iii) CaCl2 (iv) SrCl2
Answer: Option i) Beryllium haldies are essentially covalent compounds that are soluble in organic solvents such as ethanol and acetic acid.
Metals form basic hydroxides. Which of the following metal hydroxide is the least basic? (i) Mg(OH)2 (ii) Ca(OH)2 (iii) Sr(OH)2 (iv) Ba(OH)2
Answer: Option i) As the ionisation enthalpy increases from Mg to Ba, the M – O bond weakens and weakens down the group, and as a result, basicity increases down the group as a function of time. As...
Which of the carbonates given below is unstable in air and is kept in CO2 atmosphere to avoid decomposition. (i) BeCO3 (ii) MgCO3 (iii) CaCO3 (iv) BaCO3
Answer: Option i) Because of the smaller cation and larger anion sizes (smaller cation stabilises smaller anion through crystal lattice energy), Beryllium Carbonate can only be preserved in CO2...
Metal carbonates decompose on heating to give metal oxide and carbon dioxide. Which of the metal carbonates is most stable thermally? (i) MgCO3 (ii) CaCO3 (iii) SrCO3 (iv) BaCO3
Answer: Option iv) The thermal stability of metal carbonates rises as the metal's electropositive character or basicity increases from Be(OH)2 to Ba(OH)2. The most stable is BaCO3.
The reducing power of a metal depends on various factors. Suggest the factor which makes Li, the strongest reducing agent in aqueous solution. (i) Sublimation enthalpy (ii) Ionisation enthalpy (iii) Hydration enthalpy (iv) Electron-gain enthalpy
Answer: option iii) Li's hydration enthalpy is likewise high (highly exothermic). Li atom has the highest hydration enthalpy, making it the strongest reducing agent in aqueous media. - Li atom's...
Alkali metals react with water vigorously to form hydroxides and dihydrogen. Which of the following alkali metals reacts with water least vigorously? (i) Li (ii) Na (iii) K (iv) Cs
Answer: Option i) The answer is Li has a high hydrogen enthalpy. So its water reaction produces a lot of energy, which is used up infusion, vaporization, and ionization. So its water interaction is...
The alkali metals are low melting. Which of the following alkali metal is expected to melt if the room temperature rises to 30°C? (i) Na (ii) K (iii) Rb (iv) Cs
Solution: The correct answer is option (iv). The melting point of alkali metals drops as the strength of metallic bonding diminishes with increasing the size of the atom, as shown in the graph. As a...
Assertion (A): Beryllium carbonate is kept in the atmosphere of carbon dioxide. Reason (R): Beryllium carbonate is unstable and decomposes to give beryllium oxide and carbon dioxide. (i) Both A and R are correct and R is the correct explanation of A. (ii) Both A and R are correct but R is not the correct explanation of A. (iii) Both A and R are not correct. (iv) A is not correct but R is correct.
Answer: Option (i) is correct. Beryllium carbonate is stable and decomposes to give beryllium oxide and carbon dioxide. The concentration of carbon dioxide grows in the right side, causing the...
Assertion (A): The carbonate of lithium decomposes easily on heating to form lithium oxide and CO2. Reason (R): Lithium being very small in size polarises large carbonate ion leading to the formation of more stable Li2O and CO2. (i) Both A and R are correct and R is the correct explanation of A. (ii) Both A and R are correct but R is not the correct explanation of A. (iii) Both A and R are not correct (iv) A is not correct but R is correct.
Answer: Option i) is correct Lithium carbonate readily decomposes into lithium oxide and carbon dioxide. Others do not decompose. $Li _{2} CO _{3} \stackrel{\Delta}{\longrightarrow} Li _{2} O + CO...
Match the elements given in Column I with the colour they impart to the flame has given in Column II.
Column I(i) Cs (ii) Na (iii) K (iv) Ca (v) Sr (vi) Ba Column II(a) Apple green ((b) Violet (c) Brick red (d) Yellow (e) Crimson red (f) Blue Answer: (i) is f (ii) is d (iii) is b (iv) is c...
Match the compounds given in Column I with their uses mentioned in Column II.
Column I(i) CaCO3 (ii) Ca(OH)2 (iii) Cao (iv) CaSO4 Column II(a) Dentistry, ornamental work (b) Manufacture of sodium carbonate from caustic soda (c) Manufacture of high-quality paper (d) Used in...
Match the elements given in Column I with the properties mentioned in Column II.
Answer: (i) is c (ii) is b (iii) is d (iv) is a,e Explanation: Li: E is the most negative because of the extremely high hydration enthalpy. Na: NaOH is produced from the letter Na (strong base). One...
What is the structure of BeCl2 molecule in gaseous and solid-state?
Answer: The gaseous/vapour state differs from the solid state in several ways. In the solid state, BeCl2 has a polymeric chain structure, which is similar to that of water. In the presence of...
