Column (A)Column (B)(a) Plaster of Paris(i) Ca(OH)2(b) Gypsum(ii) CaSO4. ½ H2O(c) Bleaching Powder(iii)CaSO4.2H2O(d) Slaked Lime(iv) CaOCl2 Column (A)Column (B)(a) Plaster of Paris(ii) CaSO4. ½...
Match the acids given in Column (A) with their correct source given in Column (B)
Column (A)Column (B)(a) Lactic acid(i) Tomato(b) Acetic acid(ii) Lemon(c) Citric acid(iii) Vinegar(d) Oxalic acid(iv) Curd Column (A)Column (B)(a) Lactic acid(iv) Curd(b) Acetic acid(iii) Vinegar(c)...
An organic compound A on heating with concentrated H2 SO4 forms a compound B which on addition of one mole of hydrogen in presence of Ni forms a compound C. One mole of compound C on combustion forms two moles of CO2 and 3 moles of H2O. Identify the compounds A, B and C and write the chemical equations of the reactions involved.
Compound A – Ethanol (CH3CH2OH) Reaction Involved: CH3CH2OH → CH2-CH2+ H2O Compound B – Ethene (CH2= CH2) Reaction Involved: CH2=CH2 →C2H6 Compound C - Ethane (CH3 — CH3) Reaction...
Explain the given reactions with the examples
(a) Hydrogenation reaction (b) Oxidation reaction (c) Substitution reaction (d) Saponification reaction (e) Combustion reaction Answers: (a) Hydrogenation reaction - The addition reaction...
Draw the possible isomers of the compound with molecular formula C3H6O and also give their electron dot structures.
How would you bring about the following conversions? Name the process and write the reaction involved.
(a) ethanol to ethene. b) propanol to propanoic acid. Write the reactions. Answers: (a)Process of conversion: Ethanol is heated in the presence of conc. sulphuric acid at 443 K. Reaction Involved:...
Look at the figure and answer the following questions
(a) What change would you observe in the calcium hydroxide solution taken in tube B? (b) Write the reaction involved in test tubes A and B respectively. (c) If ethanol is given instead of ethanoic...
A compound C (molecular formula, C2H4O2 ) reacts with Na – metal to form a compound R and evolves a gas which burns with a pop sound. Compound C on treatment with an alcohol A in presence of an acid forms a sweet-smelling compound S (molecular formula, C3H6O2 ). On addition of NaOH to C, it also gives R and water. S on treatment with NaOH solution gives back R and A. Identify C, R, A, S and write down the reactions involved.
Identification: C - Ethanoic acid R - Sodium Ethanoate A - Ethanol S - Ester Reactions Involved: 2CH3COOH +2 Na →2CH3COONa+H2 CH3COOH +C2H5OH → CH3COOC2H5 + H20 CH3COOC2H5+NaoH...
Esters are sweet-smelling substances and are used in making perfumes. Suggest some activity and the reaction involved in the preparation of an ester with well-labelled diagram.
Activity Involved: Take 1ml ethanol and 1 ml glacial acetic acid in a test- tube. Add few drops of concentrated H2SO4. Heat the test tube for 5 minutes with the help of a boiling water bath....
Write the equations for the reactions of:
(i) Magnesium with dilute hydrochloric acid (ii) Aluminium with dilute hydrochloric acid (iii) Zinc with dilute hydrochloric acid (iv) Iron with dilute hydrochloric acid Solution: (i) Mg + 2HCl →...
(a) Write the formula and draw the electron dot structure of carbon tetrachloride.
(b) What is saponification? Write the reaction involved in this process. Answers: (a) Formula of carbon tetrachloride - CCl4 (b) Saponification is the reaction of an ester to produce sodium salt of...
Name the reaction which is commonly used in the conversion of vegetable oils to fats. Explain the reaction involved in detail.
Hydrogenation is the reaction which is commonly used in the conversion of vegetable oils to fats. In this process, Nickel acts as a catalyst. Reaction Involved:
(a) What are hydrocarbons? Give examples. (b) Give the structural differences between saturated and unsaturated hydrocarbons with two examples each. (c) What is a functional group? Give examples of four different functional groups.
Answers: (a) The Hydrocarbons are basically the compounds of Carbon and hydrogen such as Ethane and Methane. (b) The Hydrocarbons having single bond is known as saturated hydrocarbons such as Ethane...
A salt X is formed and a gas is evolved when ethanoic acid reacts with sodium hydrogen carbonate. Name the salt X and the gas evolved. Describe an activity and draw the diagram of the apparatus to prove that the evolved gas is the one which you have named. Also, write a chemical equation of the reaction involved.
Identification: Salt X - Sodium Ethanoate Gas - Carbon-di-oxide. Reaction Involved: CH3COOH+NaHCO3 → CH3COONA+H2O+CO2 Activity Involved: Experiment set up. 2. In a test-tube and take spoon full of...
What is the role of metal or reagents written on arrows in the given chemical reactions?
Write the structural formulae of all the isomers of hexane.
Match the reactions given in Column (A) with the names given in column (B).
Column AColumn B(a) CH3OH + CH3COOH CH3COOCH3 + H2O(i) Addition reaction(b) CH2 = CH2 + H2 CH3 — CH3(ii) Substitution reaction(c) CH4 + Cl2 Sunlight CH3Cl + HCl(iii) Neutralisation reaction(d)...
Unsaturated hydrocarbons contain multiple bonds between the two C-atoms and show addition reactions. Give the test to distinguish ethane from ethene.
The saturated hydrocarbons burn with a pure flame and do not produce soot while non-saturated hydrocarbons burn yellow flames and produce more soot. Ethane is saturated with hydrocarbon and heats...
Catenation is the ability of an atom to form bonds with other atoms of the same element. It is exhibited by both carbon and silicon. Compare the ability of catenation of the two elements. Give reasons.
Catenation is shown by Silicon and Carbon. Silicon bonds are not very stable and reactive but the bonds made of Carbon bonds are so strong which is why the catenation of carbon is greater than the...
In electron dot structure, the valence shell electrons are represented by crosses or dots.
(a) The atomic number of chlorine is 17. Write its electronic configuration (b) Draw the electron dot structure of chlorine molecule Answers: (a) Electronic configuration of chlorine: KLM- 2,8,7...
(c) Name the metal which is the best conductor of heat and electricity. (d) What happens when a metal reacts with dilute hydrochloric acid? Explain with the help of an example.
Solution: (c) Silver (d) Metal chloride and hydrogen are produced when a metal combines with dilute hydrochloric acid. Example: Mg + 2HCl → 2MgCl2 + H2
(a) What are metals? Name five metals. (b) Name a metal that is so soft that it can be cut with a knife.
Solution: (a) Metals are the elements that conduct electricity, are malleable and possess ductility. Example: Iron, aluminium etc. (b) Sodium
Metals are said to be shiny. Why do metals generally appear to be dull? How can their brightness be restored?
Solution: When metals are exposed to air for an extended period of time, a thin layer of oxide, carbonate, or sulphide accumulates on their surface due to the gradual action of various gases....
(a)Name two physical properties each of sodium and carbon in which their behaviour is not as expected from their classification as metal and non-metal respectively. (b)Name two metals whose melting points are so low that they melt when held in the hand.
Solution: (a) Sodium is a soft metal with a low melting point. Carbon is a non-metallic element. Graphite is a carbon allotrope that has a high melting point and is a good conductor of electricity....
Carbon, Group (14) element in the Periodic Table, is known to form compounds with many elements. Write an example of a compound formed with
(a) Chlorine (Group 17 of Periodic Table) (b) Oxygen (Group 16 of Periodic Table) Answers: (a) Compound formed with chlorine is Carbon Tetrachloride (CCl4–) (b) Compound formed with oxygen is...
Ethene is formed when ethanol at 443 K is heated with excess of concentrated sulphuric acid. What is the role of sulphuric acid in this reaction? Write the balanced chemical equation of this reaction.
Ethene is formed when ethanol at 443 K is heated with excess of concentrated sulphuric acid. Sulphuric acid helps in the formation of ethane as it is a dehydrating agent and also is a catalyst....
A gas is evolved when ethanol reacts with sodium. Name the gas evolved and also write the balanced chemical equation of the reaction involved.
Hydrogen gas is evolved when ethanol reacts with sodium. Reaction Involved: 2CH₃- CH₂-OH +2Na——>2 CH₃- CH₂- ONa + H₂
Intake of a small quantity of methanol can be lethal. Comment.
Methanol is converted into Methanal in the liver and kills all cells. Methanol also affects optic nerves and causes blindness. So, eating even small amount of methanol can be dangerous.
How is ethene prepared from ethanol? Give the reaction involved in it.
Ethene is prepared by the heating of Ethanol at 443k in the presence of excess sulphuric acid. Reaction Involved: CH3-CH2OH —> CH2=CH2 +H2O
Name the functional groups present in the following compounds
(a) CH3COCH2 CH2 CH2 CH3 (b) CH3CH2CH2COOH (c) CH3CH2 CH2 CH2 CHO (d) CH3CH2OH Identification: 1. Ketone 2. Carboxylic acid 3. Aldehyde 4....
State any five physical properties of non-metals.
Solution: Non-metallic properties Non-metallic materials are not malleable.They are brittle/non-ductile.They're usually soft.They are poor electrical conductors.They don't have a lustre to them.
Why detergents are better cleansing agents than soaps? Explain.
Detergents are better than soaps because detergents are ammonium or sulphonate salts of long-chain carboxylic acid. And, Soap forms precipitate with calcium and magnesium ions present in hard water.
A compound X is formed by the reaction of a carboxylic acid C2H4O2 and an alcohol in the presence of a few drops of H2SO4. The alcohol on oxidation with alkaline KMnO4 followed by acidification gives the same carboxylic acid as used in this reaction. Give the names and structures of (a) carboxylic acid, (b) alcohol and (c) the compound X. Also write the reaction.
(a) Ethanoic acid (CH3COOH) (b) Ethanol (CH3CH2OH) (c) Ethyl ethanoate (CH3COOCH2CH3 ) Reactions Involved: (a) (b) (c)
Identify and name the functional groups present in the following compounds.
Identification: (a) Alcohol (b) Carboxylic acid (c) Ketone (d) Alkene
Write the names of the following compounds
Identification: (a) Pentanoic acid (b) Butyne (c) Heptanal (d) Pentanol
State any five physical properties of metals.
Solution: Metal Characteristics: Metals are malleable, i.e. can be beaten into sheets. Metals can be drawn out into wires, i.e. are ductile.Metals are excellent electrical conductors.They have a...
You are given a dry cell, a torch bulb with holder, wires and crocodile clips. How would you use them to distinguish between samples of metals and non-metals?
Solution: A torch bulb is placed in a holder, and wires are linked to an electric circuit using crocodile clips. When you put a bit of sulphur between the crocodile clip and the bulb, nothing...
(a)What happens when calcium reacts with water? Write the chemical equations of the reaction of calcium with water. (b)Write the chemical equation of the reaction which takes place when iron reacts with dilute sulphuric acid. What happens when the gas produced is ignited with a burning matchstick?
Solution: (a) Calcium forms calcium hydroxide and hydrogen gas when it combines with cold water. Ca + 2H2O → Ca(OH)2 + H2 (b) Iron creates iron sulphate and hydrogen gas when it combines with dilute...
(b)What special name is given to substances like aluminium oxide. (c)Name another metal oxide that behaves like aluminium oxide.
(b) Amphoteric oxides. Act as both basic and acidic. (c) Zinc oxide is another metal that behaves like aluminium oxide.
Draw the electron dot structure of ethyne and also draw its structural formula
Electron dot structure of ethyne: Structural formula of ethyne: H-C≡C-H
The first member of the alkyne homologous series is
(a) ethyne (b) ethene (c) propyne (d) methane Answer: (b) ethyne Reason: In the alkyne series, there is a triple bond between carbon atoms. The first component of the alkyne series is ethyne with...
Write one reaction in which aluminium oxide behaves as a basic oxide and another in which it behaves as an acidic oxide.
Solution: Al2O3 + 6HCl → 2AlCl3 + 3H2O Aluminium acting as basic oxide. Al2O3 + 2NaOH → 2NaAlO2 + H2O Aluminium acting as an acidic oxide here.
Which of the following represents the saponification reaction?
(a) CH3COONa + NaOH → CH4 + Na2CO3 (b) CH3COOH + C2H5OH → CH3COOC2H5 +H2O (c) 2CH3COOH + 2Na → 2CH3COONa + H2 (d) CH3COOC2H5 + NaOH → CH3 COONa + C2H5OH Answer: (d) CH3COOC2H5 + NaOH → CH3...
The heteroatoms present in CH3 — CH2 — O — CH2— CH2 Cl are
(i) oxygen (ii) carbon (iii) hydrogen (iv) chlorine (a) (i) and (ii) (b) (ii) and (iii) (c) (iii) and (iv) (d) (i) and (iv) Answer: (d) (i) and (iv) Reason: The heteroatoms present in CH3 — CH2 — O...
The name of the compound CH3 — CH2 — CHO is
(a) Propanal (b) Propanone (c) Ethanol (d) Ethanal Answer: (a) Propanal Reason: The name of the compound CH3 — CH2 — CHO is Propanal.
Which of the following does not belong to the same homologous series?
(a) CH4 (b) C2 H6 (c) C3 H8 (d) C4 H8 Answer: (d) C4 H8 Reason: Ethane and propane are in the same series while C4 H8 is not alkene.
Which among the following are unsaturated hydrocarbons?
(a) (i) and (iii) (b) (ii) and (iii) (c) (ii) and (iv) (d) (iii) and (iv) Answer: (c) (ii) and (iv) Reason: Unsaturated hydrocarbon is a chemical compound that contains a carbon-carbon bond or...
Which of the following is not a straight-chain hydrocarbon?
Answer: (d) Reason: Option A, B, C is direct hydrocarbons, since each carbon atom is linked to one or two carbon atoms.
You are given samples of three metals- sodium, magnesium and copper. Suggest any two activities to arrange them in order of their decreasing reactivities.
Solution: The reactivity of sodium, magnesium, and copper with water can be used to sort them. When sodium comes into contact with water, it reacts violently. Magnesium and copper are two metals...
The correct electron dot structure of a water molecule is
Answer: (c) Reason: The oxygen atom has six electrons in valence, of which 2 are involved in contact with 2 hydrogen atoms. A water molecule therefore has two lone electrons joined by two bonded...
Carbon forms four covalent bonds by sharing its four valence electrons with four univalent atoms, e.g. hydrogen. After the formation of four bonds, carbon attains the electronic configuration of
(a) helium (b) neon (c) argon (d) krypton Answer: (b) neon Reason: Carbon forms four covalent bonds by sharing its four valence electrons with four univalent atoms, e.g. hydrogen. After the...
What is the action of water on aluminium? Write the equation of the chemical reaction involved.
Aluminum reacts with steam to form aluminium oxide and hydrogen gas. 2Al(s) + 3H2O(g) → Al2O3(s) + 3H2(g)
What is the action of water on (a) sodium (b) magnesium? Write equations of the chemical reactions involved.
Solution: (a) Sodium becomes sodium hydroxide and hydrogen gas when it reacts vigorously with cold water. 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g) + Heat (b) Magnesium hydroxide and hydrogen are formed...
Mineral acids are stronger acids than carboxylic acids because
(i) mineral acids are completely ionised (ii) carboxylic acids are completely ionised (iii) mineral acids are partially ionised (iv) carboxylic acids are partially ionised (a) (i) and (iv) (b) (ii)...
Vinegar is a solution of
(a) 50% – 60% acetic acid in alcohol (b) 5% – 8% acetic acid in alcohol (c) 5% – 8% acetic acid in water (d) 50% – 60% acetic acid in water Answer: (c) 5% – 8% acetic acid in water Reason:...
The correct structural formula of butanoic acid is
Answer: (d) Reason: Butanoic acid is a carboxylic acid and the structural formula of butanoic acid is shown in the option (d).
Ethanol reacts with sodium and forms two products. These are
(a) sodium ethanoate and hydrogen (b) sodium ethanoate and oxygen (c) sodium ethoxide and hydrogen (d) sodium ethoxide and oxygen Answer: (c) sodium ethoxide and hydrogen Reason: Reaction between...
(a)Give one example, with an equation, of the displacement of hydrogen by metal from an acid. (b)Name two metals (other than zinc and iron) which can displace hydrogen from dilute hydrochloric acid?
Solution: (a) Magnesium nitrate and hydrogen gas are formed when magnesium combines with very weak nitric acid. Mg(s) + 2HNO3(aq) → Mg(NO3)2(aq) + H2(g) (b) Magnesium and aluminium are two metals...
Structural formula of benzene is
Answer: (c) Reason: Benzene is a compound which is cyclic in structure having double bonds alternatively and the correct structural formula is as shown in the option (c).
Pentane has the molecular formula C5 H12. It has
(a) 5 covalent bonds (b) 12 covalent bonds (c) 16 covalent bonds (d) 17 covalent bonds Answer: (c) 16 covalent bonds Reason: Pentane has 16 covalent bonds.
In the soap micelles
(a) the ionic end of soap is on the surface of the cluster while the carbon chain is in the interior of the cluster. (b) ionic end of soap is in the interior of the cluster and the carbon chain is...
(a)Describe the reaction of potassium with water. Write the equation of the reaction involved. (b)Write an equation of the reaction of iron with steam. Indicate the physical states of all the reactants and products. (c)Which gas is produced when dilute hydrochloric acid is added to a reactive metal?
Solution: (a) Potassium creates potassium hydroxide and hydrogen gas when it reacts vigorously with cold water. 2K(s) + 2H2O(l) → 2KOH(aq) + H2(g) + Heat (b) 2Fe(s) + 4H2O(g) → Fe3O4(s) + 4H2(g) (c)...
Chlorine reacts with saturated hydrocarbons at room temperature in the
(a) absence of sunlight (b) presence of sunlight (c) presence of water (d) presence of hydrochloric acid Answer: (b) presence of sunlight Reason: Because of the halogenation process, chlorine reacts...
Identify the unsaturated compounds from the following
(i) Propane (ii) Propene (iii) Propyne (iv) Chloropropane (a) (i) and (ii) (b) (ii) and (iv) (c) (iii) and (iv) (d) (ii) and (iii) Answer: (d) (ii) and (iii) Reason: Propene and Propyne are the...
Structural formula of ethyne is
Answer: (a) Reason: Ethyne can also be known as Acetylene and is a two carbon alkyne and the correct structural formula is shown in option (a).
Which of the following is the correct representation of the electron dot structure of nitrogen?
Answer: (d) Reason: The correct representation of the electron dot structure of the nitrogen is shown in the option (d).
The soap molecule has a
(a) hydrophilic head and a hydrophobic tail (b) hydrophobic head and a hydrophilic tail (c) hydrophobic head and a hydrophobic tail (d) hydrophilic head and a hydrophilic tail Answer: (a)...
In which of the following compounds, — OH is the functional group?
(a) Butanone (b) Butanol (c) Butanoic acid (d) Butanol Answer: (b) Butanol Reason: In Butanol, –OH is the functional group.
(a)Why is sodium kept immersed in kerosene oil? (b)Why is white phosphorus kept immersed underwater? (c)Can we keep sodium immersed underwater? Why?
Solution: (a) Because sodium is a highly reactive element, it will catch fire when it comes into contact with oxygen. As a result, sodium is preserved in kerosene to prevent explosions. (a) When...
Oils on treating with hydrogen in the presence of palladium or nickel catalyst form fats. This is an example of
(a) Addition reaction (b) Substitution reaction (c) Displacement reaction (d) Oxidation reaction Answer: (a) Addition reaction Reason: Oils when treated with hydrogen in the presence of palladium or...
CH3 – CH2 – OH → CH3 – COOH (in the presence of Alkaline KMnO4+ Heat)
In the above-given reaction, alkaline KMnO4 acts as (a) reducing agent (b) oxidising agent (c) catalyst (d) dehydrating agent Answer: (b) oxidising agent Explanation: Oxidation of ethanol to form...
Which of the following are correct structural isomers of butane?
(a) (i) and (iii) (b) (ii) and (iv) (c) (i) and (ii) (d) (iii) and (iv) Answer: (c) (i) and (ii) Explanation: n−butane and iso−butane are the correct structural isomers of...
(a)Why does aluminium not react with water under ordinary conditions? (b)Name two metals that can displace hydrogen from dilute acids. (c)Name two metals that cannot displace hydrogen from dilute acids.
Solution: (a) Due to the presence of a thin coating of aluminium oxide on its surface, aluminium does not react with water under normal conditions. (b) The metals sodium and magnesium are capable of...
Buckminsterfullerene is an allotropic form of
(a) phosphorus (b) sulphur (c) carbon (d) tin Answer: (c) carbon Reason: Buckminsterfullerene is an allotropic carbon form. It has carbon atoms arranged in a ball-like pattern. It is soft, slippery,...
A molecule of ammonia (NH3 ) has
(a) only single bonds (b) only double bonds (c) only triple bonds (d) two double bonds and one single bond Answer: (a) only single bonds Explanation: An ammonia molecule has only a single...
Which of the following statements are usually correct for carbon compounds? These
(i) are good conductors of electricity (ii) are poor conductors of electricity (iii) have strong forces of attraction between their molecules (iv) do not have strong forces of attraction between...
Carbon exists in the atmosphere in the form of
(a) carbon monoxide only (b) carbon monoxide in traces and carbon dioxide (c) carbon dioxide only (d) coal Answer: (c) carbon dioxide only Reason: Carbon is a compound which is found in the...
(a)State one use of each of the following non-metals: Hydrogen, carbon (as Graphite), Nitrogen, Sulphur. (b)Name the metal which is used in making thermometers.
Solution: (a) Hydrogen is employed as rocket fuel. Electrolytic cells' electrodes are made of carbon. Nitrogen is utilised in the production of fertilisers and ammonia. Sulphur is utilised in the...
State one use of each of the following metals: Copper, Aluminium, Iron, Silver, Gold, Mercury
Solution: Copper is utilised in the production of cables and utensils. Aluminium is used in the packaging of food and some medications. Utensils are made of iron. Jewellery is made out of silver....
State five uses of non-metals.
Solution: Non-metal applications Hydrogen is employed in the production of ammonia.Hydrogen is utilised to power rockets.Electrodes are made of graphite.Because nitrogen is inert, it is utilised to...
State five uses of metals.
Solution: Metals are used for a variety of purposes. Metals are ductile, which means they can be formed into wires.Metals can be shaped, i.e. are malleable. The metal mercury is employed in...
A copper plate was dipped in AgNO3 solution. After certain time, silver from the solution was deposited on the copper plate. State the reason why it happened. Give the chemical equation of the reaction involved.
Solution: Because copper is more reactive than silver and displaces silver from the silver nitrate solution, when a copper plate is dipped in the solution, silver is deposited on the copper plate....
(a) Arrange the following metals in order of their chemical reactivity, placing the most reactive metal first: Magnesium, Copper, Iron, Sodium, Zinc, Lead, Calcium.
(b) What happens when a rod of zinc metal is dipped into a solution of copper sulphate? Give chemical equation of the reaction involved. Solution: (a)...
(a)What happens when calcium reacts with chlorine? Write an equation for the reaction which takes place. (b)What happens when magnesium reacts with very dilute nitric acid? Write an equation for the reaction involved.
Solution: (a) Calcium chloride is formed when calcium combines with chlorine to generate an ionic chloride. There is a strong reaction. Ca(s) + Cl2(g) → CaCl2(s) (b) Magnesium nitrate and hydrogen...
(a)How do metals react with hydrogen? Explain with an example. (b)How do non-metals react with hydrogen? Explain with an example.
Solution: (a) Hydrogen does not react with the majority of metals. However, some reactive metals, including sodium, potassium, calcium, and magnesium, can push hydrogen atoms to take electrons from...
(a)Explain why metals usually do not liberate hydrogen gas with dilute nitric acid.
(b)Name two metals that can, however, liberate hydrogen gas from very dilute nitric acid. Solution: (a) With dilute nitric acid, metals normally do not liberate hydrogen gas because nitric acid is a...
(a)What type of oxides are formed when non-metals react with oxygen? Explain with an example. (b)What type of oxides are formed when metals combine with oxygen? Explain with the help of an example.
(a) Acidic oxides and neutral oxides are formed when non-metals react with oxygen. Example: When carbon reacts with oxygen, it produces carbon dioxide, which is an acidic oxide, and when hydrogen...
(a)What is the nature of the oxide SO2? What happens when it is dissolved in water? Write the chemical equation of the reaction involved. (b)What is the nature of the oxide Na2O? What happens when it is dissolved in water? Write the chemical equation of the reaction involved.
Solution: (a) SO2 is acidic in nature and produces sulphurous acid when dissolved in water SO2(g) + H2O(l) → H2SO3 (aq) (b) Na2O is basic in nature and on dissolution in water produces an alkali...
(a)What are amphoteric oxides? Give two examples of amphoteric oxides.
(b)Choose the acidic oxides, basic oxides and neutral oxides from the following: Na2O; CO2.; CO; SO2; MgO; N2O; H2O (c)Which of the following are amphoteric oxides: MgO, ZnO, P2O3, Al2O3, NO2...
(b)Which of the following elements would yield: (i) an acidic oxide, (ii) a basic oxide, and (iii) a neutral oxide? Na, S, C, K, H
(b) Solutions: (i) S and C would yield acidic acidic oxides (ii) Na and K are basic oxides (iii) H would yield neutral oxide
(a)With the help of examples, describe how metal oxides differ from non-metal oxides.
Solution: (a) Metal oxides are basic in nature, turning red litmus solution blue, whereas non-metals oxides are acidic in nature, turning blue litmus solution red. For example, sodium oxide and...
Name two metals which react violently with cold water. Write any three observations you would make when such a metal is dropped into water. How would you identify the gas evolved, if any, during the reaction?
Solution: The two metals that react aggressively with cold water are sodium and potassium. Three points to consider: The reaction is exothermic, which means it produces a lot of heat. 2.Hydrogen is...
(b)Name two metals which are both malleable and ductile.
(c)Which property of iron metal is utilized in producing iron sheets required for making buckets? (d)Which property of copper metal is utilized in making thin wires? Solutions: (b) Copper and...
What is meant by saying that the metals are malleable and ductile? Explain with examples.
Because metals can be hammered into thin sheets, they are malleable. Aluminium is one example. Because metals can be stretched into wire without breaking, they are ductile. Copper is one...
Fill in the following blanks with suitable words:
(e) Calcium is a ________ reactive metal than sodium. Solution: (e) Less.
Fill in the following blanks with suitable words:
(c) Ordinary aluminium stripes are not attacked by water because of the presence of a layer of ________ on the surface of aluminium. (d) A metal having low melting point is ________ but a non-metal...
Fill in the following blanks with suitable words:
(a) Magnesium liberates ________ gas on reacting with hot boiling water. (b) The white powder formed when magnesium ribbon burns in oxygen is of ________. Solution: (a) Hydrogen. (b) Magnesium...
Complete and balance the following equations:
(c) Fe(s) + H2O → (d) Cu(NO3)(aq) + Zn → Solution: (c) Fe(s) + H2O → Fe3O4 + 4H2 (d) Cu(NO3)(aq) + Zn → Zn(NO3)2 + 2Cu
Complete and balance the following equations:
(a) Na + O2 → (b) Na2O + H2O → Solution: (a) 4Na + O2 → 2Na2O (b) Na2O + H2O → 2NaOH
Explain why, the surface of some metals acquires a dull appearance when exposed to air for a long time.
Solution: When metals are exposed to air for an extended period of time, a thin layer of oxides, carbonates, or sulphides forms on their surface, giving them a dull appearance.
Name two non-metals which are both brittle and non-ductile.
Solution: Sulphur and phosphorus are non-metals that are both brittle and non-ductile, meaning they are easily broken and flexible.
Which property of graphite is utilized in making electrodes?
Solution: Graphite has a good ability to conduct electricity. This conductivity aids in the creation of electrodes.
Name a non-metal having a very high melting point.
Solution: Diamond is a non-metal with the highest melting point of all non-metals since it is an allotrope of carbon.
Give reasons for the following: Blue colour of copper sulphate solution is destroyed when iron fillings are added to it.
Solution: Because iron is more reactive than copper, it displaces copper from copper sulphate solution, destroying the blue colour.
How would you show that silver is chemically less reactive than copper?
Solution: There will be no reaction when silver is immersed in a copper sulphate solution. Because silver cannot displace copper from copper sulphate, it has lower reactivity than copper.
What happens when iron nails are put into copper sulphate solution?
Solution: When iron nails are immersed into a copper sulphate solution, the blue colour fades and a reddish brown copper metal forms.
What will happen if a strip of copper is kept immersed in a solution of silver nitrate (AgNO3)?
Solution: When a strip of copper is immersed in a silver nitrate solution, the solution progressively becomes blue, and a gleaming greyish white deposit of silver metal forms on the copper strip.
What will happen if a strip of zinc is immersed in a solution of copper sulphate?
Solution: When zinc is submerged in a copper sulphate solution, the copper sulphate loses its blue colour and a red brown copper coating forms on the zinc.
What is meant by “brittleness”? Which type of elements usually shows brittleness: metals or non-materials?
Solution: Brittleness is a property in which something is hard but easily breakable. Brittleness can be found in almost all non-metals.
Why is there a need to harness non-conventional sources of energy? Give two main reasons.
Reasons for the need to harness non-conventional sources of energy: 1. The conventional sources are the source of energy which is depleted and will soon wear out. 2. The non-conventional energy...
Name one property which is characteristic of (a) metals, and (b) non-metals.
Solution: Non-metals are not malleable, whereas metals are.
Name the non-metal which is used: (e) In the vulcanization of rubber.
(e) Rubber vulcanization improves the rubber's strength, and sulphur is utilised in the process.
Choose the correct statement
(a) Sun can be taken as an inexhaustible source of energy (b) There is infinite storage of fossil fuel inside the earth (c) Hydro and wind energy plants are non-polluting sources of energy (d) Waste...
Name the non-metal which is used:
(c) To make electrodes of dry cells. (d) To preserve food materials. Solution: (c) Dry cell electrodes are made from carbon. (d) Nitrogen is a non-metal that is utilised to preserve food.
Name the non-metal which is used:
(a) To convert vegetable oil into vegetable ghee (solid fat). (b) As a rocket fuel (in liquid form). Solution: (a) Hydrogen is a non-metal that is utilised to transform vegetable oil into ghee. (b)...
Which metal foil is used for packing some of the medicine tablets?
Some pharmaceutical tablets, as well as food items, are packaged in aluminium foil.
Name two metals which are used: For making jewellery and to decorate sweets.
Metals such as gold and silver are used to make jewellery and to embellish desserts.
Name two metals which are used:
(a)For making electric wires. (b)For making domestic utensils and factory equipment. Solution: (a) The metals utilised to make electric cables are aluminium and copper. (a) Copper and aluminium are...
Acid rain happens because
(a) sun leads to heating of the upper layer of the atmosphere (b) burning of fossil fuels release oxides of carbon, nitrogen and sulphur in the atmosphere (c) electrical charges are produced due to...
Write equation for the reaction of:
(a)Sodium with oxygen (b)Magnesium with oxygen Solution: (a) When sodium combines with oxygen, sodium oxide is formed. 4Na(s) + O2(g) → 2Na2O(s) (b) Magnesium oxide is formed when magnesium combines...
(a)Name one metal which is stored in kerosene oil. (b)Name one non-metal which is stored under water.
Solution: (a) Sodium is a metal that is held in kerosene oil to keep it from reacting with oxygen and moisture. (b) White phosphorus is a non-metal that is kept immersed in water. When exposed to...
Which of the two metals is more reactive: copper or silver?
Solution: Copper has a higher level of reactivity than silver. The less reactive element can be displaced from the solution by the more reactive element.
Name the metal which has been placed:
(a)At the bottom of the reactivity series (b)At the top of the reactivity series (c)Just below copper in the reactivity series Solution: (a) Gold is the metal at the bottom of the reactivity series...
Write the names and formulae of (a) a metal hydride, and (b) a non-metal hydride.
Solutions: An example of a metal hydride is Sodium hydride(NaH). An example of a non-metal hydride is Hydrogen sulphide(H2S).
Which property of copper and aluminium makes them suitable:
(a)For making cooking utensils and boilers? (b)For making electric wires? Solution: (a)Copper and aluminium have excellent thermal conductivity, making them ideal for use in cooking equipment and...
A copper coin is kept immersed in a solution of silver nitrate for some time. What will happen to the coin and the colour of the solution?
Solution: When a copper coin is immersed in a silver nitrate solution for a period of time, the coin's colour changes to a gleaming greenish-white due to the deposition of silver on it.
Name two metals which form amphoteric oxides.
Solution: Aluminum and zinc are amphoteric oxides that have both basic and acidic properties.
What name is given to those metal oxides which show basic as well as acidic behaviour?
Solution: Metal oxides that exhibit both acidic and basic behaviour are known as amphoteric oxides.
Give the names and formulae of (a) two acidic oxides, and (b) two basic oxides.
Solution: (a)The two acidic oxides are carbon dioxide (CO2) and sulphur dioxide (SO2). (b) The basic oxides sodium oxide (Na2O) and magnesium oxide (MgO) are two examples.
What is aqua-regia? Name two special metals which are insoluble in common reagents but dissolve in aqua-regia.
Solution: A freshly made mixture of one part concentrated nitric acid and three parts concentrated hydrochloric acid is known as aqua-regia. Gold and platinum are rare metals that are insoluble in...
What changes in the colour of iron nails and copper sulphate solution do you observe after keeping the iron nails dipped in copper sulphate solution for about 30 minutes?
A reddish brown coating of copper metal is applied to an iron nail. Copper is displaced from copper sulphate solution by an iron nail, resulting in ferrous sulphate, and the colour of copper...
A student-focussed the image of a candle flame on a white screen using a convex lens. He noted down the position of the candle screen and the lens as under
Position of candle = 12.0 cm Position of convex lens = 50.0 cm Position of the screen = 88.0 cm What is the focal length of the convex lens?Where will the image be formed if he shifts the candle...
Define power of a lens. What is its unit? One student uses a lens of focal length 50 cm and another of –50 cm. What is the nature of the lens and its power used by each of them?
Power of lens Is the degree of convergence and divergence provided by a lens and its unit is Diopter (D). The focal length of the lens used by the first student is in positive so it is a convex...
From amongst the metals sodium, calcium, aluminium, copper and magnesium, name the metal:
(i)Which reacts with water only on boiling, and (ii)Another which does not react even with steam. Solution: (I) Magnesium reacts with boiling water only. (ii) Even steam has no effect on...
State whether the following statement is true or false: Non-metals react with dilute acids to produce a gas which burns with a pop sound.
False. When metals react with dilute acids, hydrogen gas is produced, which ignites with a pop sound.
Size of the image of an object by a mirror having a focal length of 20 cm is observed to be reduced to 1/3rd of its size. At what distance the object has been placed from the mirror? What is the nature of the image and the mirror?
Given, m = 1/3 Using = 1/v+1/u=1/f Calculating u, u = – 80 cm. The image is real and inverted so the mirror is concave.
The image of a candle flame formed by a lens is obtained on a screen placed on the other side of the lens. If the image is three times the size of the flame and the distance between the lens and image is 80 cm, at what distance should the candle be placed from the lens? What is the nature of the image at a distance of 80 cm and the lens?
Ray Diagram The image in the screen is real and so, the magnification is, m = –3 v = 80 cm u = ? m = v/u –3 = 80/u u = –80/3 cm As we know, 1/f = 1/v – 1/u =1/80 + 3/80 = 4/80 =...
Draw ray diagrams showing the image formation by a convex mirror when an object is placed
(a) at infinity (b) at a finite distance from the mirror (a) (b)
Name the metal which is the poorest conductor of heat.
Lead is a metal that has a low heat conductivity.
Draw ray diagrams showing the image formation by a concave lens when an object is placed
(a) at the focus of the lens (b) between focus and twice the focal length of the lens (c) beyond twice the focal length of the lens (a) (b) (c)
Write laws of refraction. Explain the same with the help of a ray diagram, when a ray of light passes through a rectangular glass slab.
The Laws of refraction: The Incident ray, refracted ray and the normal at the point of incidence lies in the same plane. The ratio of the sine of incidence and sine of refraction is same for the...
Name one metal which has a low melting point.
Cesium is a metal with a relatively low melting point.
Draw ray diagrams showing the image formation by a convex lens when an object is placed
(a) between optical centre and focus of the lens (b) between focus and twice the focal length of the lens (c) at twice the focal length of the lens (d) at infinity (e) at the focus of the lens (a)...
Draw ray diagrams showing the image formation by a concave mirror when an object is placed
(a) between pole and focus of the mirror (b) between focus and centre of curvature of the mirror (c) at the centre of curvature of the mirror (d) a little beyond the centre of curvature of the...
Draw a ray diagram showing the path of rays of light when it enters with oblique incidence
1. From air into water; 2. From water into air. 1. 2.
Under what condition in an arrangement of two plane mirrors, incident ray and reflected ray will always be parallel to each other, whatever may be the angle of incidence. Show the same with the help of a diagram.
When two plane mirrors are placed perpendicular to each other, then the Incident ray and the reflected ray will always be parallel to each other.
How are power and focal length of a lens related? You are provided with two lenses of focal length 20 cm and 40 cm respectively. Which lens will you use to obtain more convergent light?
The power of lens is inversely proportional to the focal length of the lens and the lens with focal length 20 has more power when compared to a lens with a focal length of 40 cm. The lens having...
Refractive index of diamond with respect to glass is 1.6 and the absolute refractive index of glass is 1.5. Find out the absolute refractive index of diamond.
Given, Absolute RI of diamond= 1.6 Absolute RI of glass= 1.5 Thus, Absolute refractive index of diamond = 2.4
Sudha finds out that the sharp image of the window pane of her science laboratory is formed at a distance of 15 cm from the lens. She now tries to focus the building visible to her outside the window instead of the window pane without disturbing the lens. In which direction will she move the screen to obtain a sharp image of the building? What is the approximate focal length of this lens?
To get a clear picture of the structure, Sudha has to move the screen and remove the lens. The length of the area will be about 15 cm. The light rays from a distant object such as a tree or a...
A convex lens of focal length 20 cm can produce a magnified virtual as well as real image. Is this a correct statement? If yes, where shall the object be placed in each case for obtaining these images?
When an object is in F and F2 of a convex lens. So, we need to place the object between 20 and 40 cm of the lens. When an object is placed between F and 0 of a convex lens, its magnified, vertical...
How is the refractive index of a medium related to the speed of light? Obtain an expression for the refractive index of a medium with respect to another in terms of speed of light in these two media?
The Refractive Index can be seen as a factor in reducing the speed and length of radiation in relation to vacuum values. w = civ where, n- Refractive index c – Speed of light v- Velocity The ratio...
A pencil, when dipped in water in a glass tumbler, appears to be bent at the interface of air and water. Will the pencil appear to be bent to the same extent, if instead of water we use liquids like, kerosene or turpentine. Support your answer with reason.
The bending of light here is a reversal function. Reversal is based on refractive indices. The opposing indices of paraffin or turpentine will not be the same as water. So the degree of bending will...
(a)Name the most abundant metal in the earth’s crust. (b)Name the most abundant non-metal in the earth’s crust.
Solution: (a)The most abundant metal in the earth's crust is aluminium. (b)In the earth's crust, oxygen is the most abundant non-metal.
Why does a light ray incident on a rectangular glass slab immersed in any medium emerges parallel to itself? Explain using a diagram.
When light ray penetrates into the denser medium from the abnormal bend to the normal In this case the magnitude of the curvature of the parallel radiation is the same. Thus, the emergent ray is...
Identify the device used as a spherical mirror or lens in the following cases, when the image formed is virtual and erect in each case.
1. Object is placed between the device and its focus, an image formed is enlarged and behind it. 2. Object is placed between the focus and device, an image formed is enlarged and on the same side as...
In which of the following, the image of an object placed at infinity will be highly diminished and point sized?
(a) Concave mirror only (b) Convex mirror only (c) Convex lens only (d) Concave mirror, convex mirror, concave lens and convex lens Answer: (d) Concave mirror, convex mirror, concave lens and convex...
A child is standing in front of a magic mirror. She finds the image of her head bigger, the middle portion of her body of the same size and that of the legs smaller. The following is the order of combinations for the magic mirror from the top.
(a) Plane, convex and concave (b) Convex, concave and plane (c) Concave, plane and convex (d) Convex, plane and concave Answer: (c) Concave, plane and convex Explanation: Concave mirror showed her...
Which of the following ray diagrams is correct for the ray of light incident on a lens shown in the figure?
(a) Fig. A. (b) Fig. B. (c) Fig. C. (d) Fig. D. Answer: (a) Fig. A. Explanation: The ray diagram in figure A is correct for the light ray incident on a lens as the incident ray travels through the...
Why are metals called electropositive elements whereas non-metals are called electronegative elements?
Metals are considered electropositive because they can lose electrons from an atom to generate positive ions, while non-metals are called electronegative because they can absorb electrons and form...
You are given water, mustard oil, glycerine and kerosene. In which of these media a ray of light incident obliquely at same angle would bend the most?
(a) Kerosene (b) Water (c) Mustard oil (d) Glycerine Answer: (d) Glycerine Explanation: A ray of light incident obliquely at same angle would bend the most in glycerine media.
Which of the following ray diagrams is correct for the ray of light incident on a concave mirror as shown in the figure?
LRR PIC 6----- (a) Fig. A (b) Fig. B (c) Fig. C (d) Fig. D Answer: (d) Fig. D Explanation: The ray diagram in Figure D is correct for the ray of light incident on a concave mirror as the incident is...
The path of a ray of light coming from air passing through a rectangular glass slab traced by four students are shown as A, B, C and D in the Figure. Which one of them is correct?
(a) A (b) B (c) C (d) D Answer: b) B Explanation: The path of a ray of light coming from air passing through a rectangular glass slab traced by the student B is correct.
The laws of reflection hold good for
(a) plane mirror only (b) concave mirror only (c) convex mirror only (d) all mirrors irrespective of their shape Answer: (d) all mirrors irrespective of their shape Explanation: The laws of...
In torches, searchlights and headlights of vehicles the bulb is placed
(a) between the pole and the focus of the reflector (b) very near to the focus of the reflector (c) between the focus and centre of curvature of the reflector (d) at the centre of curvature of the...
Name one metal and one non-metal which exist in liquid state at room temperature.
Mercury is a metal, and bromine is a nonmetal that exists in liquid form at room temperature.
A full-length image of a distant tall building can definitely be seen by using
(a) a concave mirror (b) a convex mirror (c) a plane mirror (d) both concave as well as plane mirror Answer: (b) a convex mirror Explanation: A full-length image of a distant tall building can...
Rays from Sun converge at a point 15 cm in front of a concave mirror. Where an object should be placed so that size of its image is equal to the size of the object?
(a) 15 cm in front of the mirror (b) 30 cm in front of the mirror (c) between 15 cm and 30 cm in front of the mirror (d) more than 30 cm in front of the mirror Answer: (d) more than 30 cm in front...
Magnification produced by a rearview mirror fitted in vehicles
(a) is less than one (b) is more than one (c) is equal to one (d) can be more than or less than one depending upon the position of the object in front of it Answer: (a) is less than one Explanation:...
Which of the following statements is true?
(a) A convex lens has 4 dioptre power having a focal length 0.25 m (b) A convex lens has –4 dioptre power having a focal length 0.25 m (c) A concave lens has 4 dioptre power having a focal length...
A beam of light is incident through the holes on side A and emerges out of the holes on the other face of the box as shown in the figure. Which of the following could be inside the box?
(a) Concave lens (b) Rectangular glass slab (c) Prism (d) Convex lens Answer: (d) Convex lens Explanation: When a beam of light is incident through the holes on side A and emerges out of the holes...
Name the following halides according to the IUPAC system and classify them as alkyl, allyl, benzyl ( primary, secondary, tertiary ), vinyl, or aryl halides
(i) CH3C(Cl)(C2H5)CH2CH3 (ii) CH3CH=C(Cl)CH2CH(CH3)2 Solution: (i) 3−Chloro−3–methylpentane (Tertiary alkyl halide) (ii) 3−Chloro−5−methylhex−2−ene (Vinyl halide)
Beams of light are incident through the holes A and B and emerge out of the box through the holes C and D respectively as shown in the Figure. Which of the following could be inside the box?
(a) A rectangular glass slab (b) A convex lens (c) A concave lens (d) A prism Answer: (a) A rectangular glass slab Explanation: When the beams of light are incident through the holes A and B and...
A light ray enters from medium A to medium B as shown in the Figure. The refractive
index of medium B relative to A will be (a) greater than unity (b) less than unity (c) equal to unity (d) zero Answer: (a) greater than unity Explanation: The refractive index is the bending of a...
The figure shows a ray of light as it travels from medium A to medium B. Refractive index of the
medium B relative to medium A is (a) √3 / √2 (b) 2 / 3 (c) 1/ 2 (d) 2 Answer: (a) √3 / √2 Explanation: According to the Snell’s law, the refractive index of the medium B relative to the medium A is...
Under which of the following conditions a concave mirror can form an image larger than the actual object?
(a) When the object is kept at a distance equal to its radius of curvature (b) When an object is kept at a distance less than its focal length (c) When an object is placed between the focus and...
A 10 mm long awl pin is placed vertically in front of a concave mirror. A 5 mm long image of the awl pin is formed at 30 cm in front of the mirror. The focal length of this mirror is
(a) – 30 cm (b) – 20 cm (c) – 40 cm (d) – 60 cm Answer: (b) – 20 cm Explanation: In this case, the focal length of the concave mirror is – 20 cm.
Which of the following can make a parallel beam of light when light from a point source is incident on it?
(a) Concave mirror as well as convex lens (b) Convex mirror as well as a concave lens (c) Two plane mirrors placed at 90° to each other (d) Concave mirror as well as a concave lens Answer: (d)...
Atomic number of a few elements are given below 10, 20, 7, 14
(a) Identify the elements
Solution: (a)Elements are Neon (10) Calcium (20) Nitrogen (7) Silicon (14) (b)Neon belongs to group 18, Calcium belongs to group 2, Nitrogen belongs to group 7 and Silicon belongs to group 14.
An element is placed in 2nd Group and 3rd Period of the Periodic Table, burns in presence of oxygen to form a basic oxide.
(a) Identify the element
(b) Write the electronic configuration.
Solution: The answer is: (a) Element is Magnesium (b) Electronic Configuration-2,8,2
Give an account of the process adopted by Mendeleev ′ for the classification of elements. How did he arrive at “Periodic Law”?
Solution: Only 63 elements were known at the time of Mendeleev's discovery. Mendeleev looked into the relationship between chemical characteristics and the atomic manes of elements in the periodic...
Which group of elements could be placed in Mendeleev’s ′ Periodic Table without disturbing the original order? Give reason.
Solution: Inert gases could be included in Mendeleev's Periodic Table without causing any disruptions to the original order of elements. Noble gases, such as Helium, were known prior to Mendeleev's...
Monochromatic light of wavelength 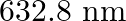 is produced by a helium-neon laser. The power emitted is
is produced by a helium-neon laser. The power emitted is 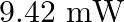 . How fast does a hydrogen atom have to travel in order to have the same momentum as that of the photon?
. How fast does a hydrogen atom have to travel in order to have the same momentum as that of the photon?
We are provided that the momentum of the hydrogen atom is equal to the momentum of the photon, $\mathrm{P}=1.047 \times 10^{-27} \mathrm{~kg} \mathrm{~m} / \mathrm{s}$ Momentum's expression is: $P=m...
Monochromatic light of wavelength 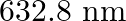 is produced by a helium-neon laser. The power emitted is
is produced by a helium-neon laser. The power emitted is 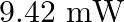 (a) Find the energy and momentum of each photon in the light beam
(a) Find the energy and momentum of each photon in the light beam
(b) How many photons per second, on the average, arrive at a target irradiated by this beam? (Assume the beam to have a uniform cross-section which is less than the target area)
Wavelength of a monochromatic light is given as $\lambda=632.8 \mathrm{~nm}=632.8 \times 10^{-9} \mathrm{~m}$ Power emitted by the laser is, $P=9.42 \mathrm{~mW}=9.42 \times 10^{-3} \mathrm{~W}$...
Three elements B, Si and Ge are
(a) metals
(b) non-metals
(c) metalloids
(d) metal, non-metal, and metalloid respectively
Solution: The answer is (c) metalloids Explanation: Metalloids include the elements boron (B), silicon (Si), and germanium (Ge). Arsenic (As), antimony (Sb), tellurium (Te), polonium (Po), and...
Arrange the following elements in the order of their increasing non-metallic character Li, O, C, Be, F
(a) F < O < C < Be < Li
(b) Li < Be < C < O< F
(c) F < O < C < Be < Li
(d) F < O < Be < C < Li
Solution: The answer is b) Explanation: Li is located on the left side of the current periodic table in the second period, followed by berrylium. Fluorine is located directly across from Neon. As a...
Which of the following are the characteristics of isotopes of an element?
(i) Isotopes of an element have the same atomic masses
(ii) Isotopes of an element have the same atomic number
(iii) Isotopes of an element show the same physical properties
(iv) Isotopes of an element show the same chemical properties
(a) (i), (iii) and (iv)
(b) (ii), (iii) and (iv)
(c) (ii) and (iii)
(d) (ii) and (iv)
Solution: Option d) is the answer. Explanation: Isotopes are elements that have the same atomic number but have different atomic masses, and they are classified as such. Isotopes have the same...
Which of the following elements does not lose an electron easily?
(a) Na
(b) F
(c) Mg
(d) Al
Solution: Option b) is the answer Explanation: Sodium has one electron, magnesium has two, and aluminum has three electrons in its outermost shell, whereas fluorine has seven electrons in its...
Which of the following elements would lose an electron easily?
(a) Mg
(b) Na
(c) K
(d) Ca
Solution: The answer is (c) K Explanation: Magnesium and calcium are both members of the group I, while sodium and potassium are members of a different group (II). Given that K has only one electron...
Which among the following elements has the largest atomic radii?
(a) Na
(b) Mg
(c) K
(d) Ca
Solution: The answer is (c) K Explanation: Because atomic radii decrease from left to right during a period of time, K has the biggest atomic radii of all the elements. This is due to an increase in...
The elements A, B, C, D and E have atomic number 9, 11, 17, 12 and 13 respectively. Which pair of elements belong to the same group?
(a) A and B
(b) B and D
(c) A and C
(d) D and E
Solution: The answer is (c) A and C Explanation: Here A is Fluorine(Group 17), B is Sodium (Group 1), C is Chlorine (Group 17), D is Magnesium(Group 2) and E is Aluminium (Group 13). A and C are...
Which of the following statements about the Modern Periodic Table is correct:
(a) It has 18 horizontal rows known as Periods
(b) It has 7 vertical columns known as Periods
(c) It has 18 vertical columns known as Groups
(d) It has 7 horizontal rows known as Groups
Solution: The answer is (c) It has 18 vertical columns known as Groups Explanation: The modern periodic table is divided into 18 groups and seven periods. Columns are referred to as groups, while...
In Mendeleev’s Periodic Table, gaps were left for the elements to be discovered later. Which of the following elements found a place in the periodic table later
(a) Germanium
(b) Chlorine
(c) Oxygen
(d) Silicon
Solution: Answer: a) Germanium Explanation: Initially, Mendeleev designated unidentified elements as EKA- Boron, EKA- Aluminum, and EKA Silicon, which were later renamed Scandium, Gallium, and...
Up to which element, the Law of Octaves was found to be applicable
(a) Oxygen
(b) Calcium
(c) Cobalt
(d) Potassium
Solution: Answer: Option (b) Calcium Explanation: Newland's law of octaves is applicable to elements with atomic masses up to 40 da, which corresponds to the elements up to and including Calcium....
The photoelectric cut-off voltage in a certain experiment is 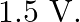 What is the maximum kinetic energy of photoelectrons emitted?
What is the maximum kinetic energy of photoelectrons emitted?
Photoelectric cut-off voltage is given as $\mathbf{V}_{\mathbf{0}}=\mathbf{1 . 5} \mathbf{V}$ The maximum kinetic energy for emitted photoelectrons, $K_{e}=eV$ Where, $e=$ charge on an electron...
The work function of caesium metal is 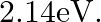 When light of frequency
When light of frequency 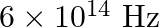 is incident on the metal surface, photoemission of electrons occurs. What is the maximum speed of the emitted photoelectrons?
is incident on the metal surface, photoemission of electrons occurs. What is the maximum speed of the emitted photoelectrons?
$\mathbf{v}$ is the maximum speed of photoelectrons emittedThe kinetic energy relation can be written as:$$\mathrm{K}=\frac{1}{2} m v^{2}$$Where,$$\mathrm{m}=\text { mass of electron }=9.1 \times...
The work function of caesium metal is 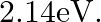 When light of frequency
When light of frequency 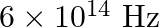 is incident on the metal surface, photoemission of electrons occurs. What is the (a) maximum kinetic energy of the emitted electrons (b) Stopping potential
is incident on the metal surface, photoemission of electrons occurs. What is the (a) maximum kinetic energy of the emitted electrons (b) Stopping potential
Work function of caesium is given as $\Phi_{o}=2.14 \mathrm{eV}$ Frequency of light is given as $\mathbf{v}=6.0 \times 10^{14} \mathrm{~Hz}$ (a) The maximum energy (kinetic) by the photoelectric...
Nerve center is a super expert endocrine organ. Elaborate.
solution: Nerve center is a super expert endocrine organ as it secretes chemicals that control the blend and discharge of the pituitary organ which is the expert organ. It is associated with the...
Outline the contrasts between the instrument of activity of a protein and a steroid chemical.
solution: Protein chemical: Protein chemicals are insulin, glucagon, prolactin and so on They need to tie their receptors which are available on the plasma layer. They structure their chemical...
Calcium assumes a vital part in the development of bones. Compose on the job of endocrine organs and chemicals answerable for keeping up with Calcium homeostasis.
solution: Thyroid organ and the parathyroid organ that is a main adversary with one another are answerable for keeping up with calcium homeostasis. Calcitonin is answerable for the high calcium...
An example of pee was analyzed to contain a high substance of glucose and ketone bodies. In light of this perception, answer the accompanying:
a. Which endocrine organ and chemical is identified with this condition? b. Name the cells on which this chemical demonstrations. c. What is the condition called and how might it be corrected?...
