False, Defense: L.H.S \[=\text{ }\left( tan\text{ }\theta +2 \right)\text{ }\left( 2\text{ }tan\text{ }\theta +1 \right)\] \[=\text{ }2\text{ }tan2\text{ }\theta \text{ }+\text{ }tan\text{ }\theta...
If cosA + cos2A = 1, then sin2A + sin4A = 1.
True: Reason: As indicated by the inquiry, \[cos\text{ }A+cos2\text{ }A\text{ }=\text{ }1\] i.e., \[cos\text{ }A\text{ }=\text{ }1-cos2\text{ }A\] Since, \[sin2\text{ }\theta +cos2\text{ }\theta...
√((1– cos2θ) sec2 θ)= tan θ
Solution: True, reason:
The value of the expression (sin 80° – cos 80°) is negative.
False, Defense: We realize that, sin θ increments when 0° ≤ θ ≤ 90° cos θ diminishes when 0° ≤ θ ≤ 90° What's more, (sin 80°-cos 80°) = (expanding esteem diminishing worth) = a positive worth....
The value of the expression (cos223° – sin267°) is positive.
False, Reason: Since, \[\left( a2-b2 \right)\text{ }=\text{ }\left( a+b \right)\left( a-b \right)\] \[cos2\text{ }23{}^\circ \text{ }\text{ }sin2\text{ }67{}^\circ \text{ }=\left( cos\text{...
Write ‘True’ or ‘False’ and justify your answer in each of the following:
1. tan 47o/cot 43 ° = 1 Valid Legitimization: Since, tan (90° - θ) = bed θ
If cos 9α = sinα and 9α < 90°, then the value of tan5α is
(A) 1/√3 (B) √3 (C) 1 (D) 0 (C) 1 As indicated by the inquiry, \[cos\text{ }9\propto =\text{ }sin\propto and\text{ }9\propto <90{}^\circ \] for example 9α is an intense point We realize that,...
The value of (tan1° tan2° tan3° … tan89°) is
(A) 0 (B) 1 (C) 2 (D) ½ (B) 1 tan 1°. tan 2°.tan 3° … tan 89° =tan1°.tan 2°.tan 3°… tan 43°.tan 44°.tan 45°.tan 46°.tan 47°… tan 87°.tan 88°.tan 89° Since, tan 45° = 1, = tan1°.tan 2°.tan 3°… tan...
If cos (α + β) = 0, then sin (α – β) can be reduced to
(A) cos β (B) cos 2β (C) sin α (D) sin 2α (B) cos 2β As indicated by the inquiry, \[cos\left( \alpha +\beta \right)\text{ }=\text{ }0\] Since, cos 90° = 0 We can compose, \[cos\left( \alpha...
Given that sinθ = a b , then cosθ is equal to
(A) b/√(b2– a2) (B) b/a (C) √(b2-a2)/b (D) a/√(b2-a2) (C) √(b2 – a2)/b As indicated by the inquiry, \[sin\text{ }\theta \text{ }=a/b\] We know, \[sin2\text{ }\theta \text{ }+cos2\text{ }\theta...
The value of the expression [cosec (75° + θ) – sec (15° – θ) – tan (55° + θ) + cot (35° – θ)] is
(A) – 1 (B) 0 (C) 1 (D) 3 2 (B) 0 As indicated by the inquiry, We need to discover the worth of the situation, \[cosec\left( 75{}^\circ +\theta \right)\text{ }\text{ }sec\left( 15{}^\circ...
If sin A = ½ , then the value of cot A is
(A) √3 (B) 1/√3 (C) √3/2 (D) 1 (A) √3 As indicated by the inquiry, \[Sin\text{ }A\text{ }=\text{ }{\scriptscriptstyle 1\!/\!{ }_2}\text{ }\ldots \text{ }\left( 1 \right)\] We realize that, NCERT...
Choose the correct answer from the given four options: If cos A = 4/5, then the value of tan A is
(A) 3/5 (B) ¾ (C) 4/3 (D) 5/3 (B) 3/4 As indicated by the inquiry, \[cos\text{ }A\text{ }=\text{ }4/5\text{ }\ldots \text{ }\left( 1 \right)\] We know, \[tan\text{ }A\text{ }=\text{ }sinA/cosA\] To...
On the off chance that Zeba were more youthful by 5 years than what she truly is, the square of her age (in years) would have been 11 in excess of multiple times her real age. What is her age now?
Allow Zeba's to age = x As indicated by the inquiry, \[\left( x-5 \right){}^\text{2}=11+5x\] \[x{}^\text{2}+25-10x=11+5x\] \[x{}^\text{2}-15x+14=0\] \[x{}^\text{2}-14x-x+14=0\] \[x\left( x-14...
A train, going at a uniform speed for 360 km, would have required 48 minutes less to venture to every part of a similar distance if its speed were 5 km/h more. Track down the first speed of the train.
Let unique speed of train = x km/h We know, Time = distance/speed As indicated by the inquiry, we have, Time taken via train = 360/x hour What's more, Time taken via train its speed increment 5 km/h...
Find a natural number whose square diminished by 84 is equal to thrice of 8 more than the given number.
Let the normal number = 'x'. As per the inquiry, We get the condition, \[x{}^\text{2}\text{ }\text{ }84\text{ }=\text{ }3\left( x+8 \right)\] \[x{}^\text{2}\text{ }\text{ }84\text{ }=\text{...
Find the roots of the quadratic equations by using the quadratic formula in each of the following:
(½)x2– √11x + 1 = 0 using the formulae: we get,
Find the roots of the quadratic equations by using the quadratic formula in each of the following:
x2 + 2 √2x – 6 = 0x2 – 3 √5x + 10 = 0 using formulae: 5. we get, 6.
Find the roots of the quadratic equations by using the quadratic formula in each of the following:
–3x2 + 5x + 12 = 0–x2 + 7x – 10 = 0 using the formulae: 3. we get, 4.
Find the roots of the quadratic equations by using the quadratic formula in each of the following:
2 x2 – 3x – 5 = 05x2 + 13x + 8 = 0by using ths formulae below: 1. we get, 2.
If PA and PB are tangents from an outside point P. such that PA = 10 cm and ∠APB = 60°. Find the length of chord AB.
Given, $AP=10cm$ $\angle APB={{60}^{\circ }}$ According to the figure We know that, A line drawn from centre to point from where external tangents are drawn, bisects the angle made by tangents at...
A quadratic equation with integral coefficient has integral roots. Justify your answer.
No, a quadratic condition with essential coefficients might possibly have indispensable roots. Support Think about the accompanying condition, \[8x2\text{ }\text{ }2x\text{ }\text{ }1\text{ }=\text{...
Write whether the following statements are true or false. Justify your answers.
If the coefficient of x2 and the constant term of a quadratic equation have opposite signs, then the quadratic equation has real roots.If the coefficient of x2 and the constant term have the same...
Write whether the following statements are true or false. Justify your answers.
Every quadratic equation has at least two roots.Every quadratic equations has at most two roots. (iii) False. For instance, a quadratic condition \[x2\text{ }\text{ }4x\text{ }+\text{ }4\text{...
Write whether the following statements are true or false. Justify your answers.
Every quadratic equation has exactly one root.Every quadratic equation has at least one real root. (I) False. For instance, a quadratic condition \[x2\text{ }\text{ }9\text{ }=\text{ }0\] has two...
State whether the following quadratic equations have two distinct real roots. Justify your answer.
(x – 1) (x + 2) + 2 = 0(x + 1) (x – 2) + x = 0 (ix) The condition (x – 1) (x + 2) + 2 = 0 has two genuine and unmistakable roots. Working on the above condition, \[x2\text{ }\text{ }x\text{ }+\text{...
State whether the following quadratic equations have two distinct real roots. Justify your answer.
√2 x2 –(3/√2)x + 1/√2 = 0x (1 – x) – 2 = 0 (vii) The condition √2x2 – 3x/√2 + ½ = 0 has two genuine and unmistakable roots. \[\begin{array}{*{35}{l}} D\text{ }=\text{ }b2\text{ }\text{...
State whether the following quadratic equations have two distinct real roots. Justify your answer.
(x + 4)2 – 8x = 0(x – √2)2 – 2(x + 1) = 0 (v) The condition (x + 4)2 – 8x = 0 has no genuine roots. Working on the above condition, \[x2\text{ }+\text{ }8x\text{ }+\text{...
State whether the following quadratic equations have two distinct real roots. Justify your answer.
2x2 – 6x + 9/2 = 03x2 – 4x + 1 = 0 (iii) The condition 2x2 – 6x + (9/2) = 0 has genuine and equivalent roots. \[D\text{ }=\text{ }b2\text{ }\text{ }4ac\] \[=\text{ }\left( -\text{ }6 \right)2\text{...
State whether the following quadratic equations have two distinct real roots. Justify your answer.
x2 – 3x + 4 = 02x2 + x – 1 = 0 (I) The condition x2 – 3x + 4 = 0 has no genuine roots. \[D\text{ }=\text{ }b2\text{ }\text{ }4ac\] \[=\text{ }\left( -\text{ }3 \right)2\text{ }\text{...
Which of the following equations has the sum of its roots as 3?
(A) 2x2 – 3x + 6 = 0 (B) –x2 + 3x – 3 = 0 (C) √2x2 – 3/√2x+1=0 (D) 3x2 – 3x + 3 = 0 (B) – \[x2\text{ }+\text{ }3x\text{ }\text{ }3\text{ }=\text{ }0\] The amount of the foundations of a...
In Fig below, PQ is tangent at point R of the circle with center O. If ∠TRQ = 30°, find ∠PRS
Given, $\angle TRQ={{30}^{\circ }}$ . At point R, OR ⊥ RQ. So, $\angle ORQ={{90}^{\circ }}$ $\Rightarrow \angle TRQ+\angle ORT={{90}^{\circ }}$ $\Rightarrow \angle ORT={{90}^{\circ...
If ½ is a root of the equation x2 + kx – 5/4 = 0, then the value of k is
(A) 2 (B) – 2 (C) ¼ (D) ½ (A) 2 In case ½ is a foundation of the situation \[x2\text{ }+\text{ }kx\text{ }\text{ }5/4\text{ }=\text{ }0\] then, at that point, subbing the worth of ½ instead of x...
Which of the following equations has 2 as a root?
(A) x2 – 4x + 5 = 0 (B) x2 + 3x – 12 = 0 (C) 2x2 – 7x + 6 = 0 (D) 3x2 – 6x – 2 = 0 (C) \[2x2\text{ }\text{ }7x\text{ }+\text{ }6\text{ }=\text{ }0\] Assuming 2 is a...
Which of the following is not a quadratic equation?
(A) 2(x – 1)2 = 4x2 – 2x + 1 (B) 2x – x2 = x2 + 5 (C) (√2x + √3)2 + x2 = 3x2 − 5x (D) (x2 + 2x)2 = x4 + 3 + 4x3 (D) \[\left( x2\text{ }+\text{ }2x...
Prove that the intercept of a tangent between two parallel tangents to a circle subtends a right angle at centre.
We are considering a circle with centre ‘O’ with two parallel tangents through A & B at ends of diameter. M intersect the parallel tangents at P and Q Then, required to prove: $\angle...
If AB, AC, PQ are the tangents in the figure, and AB = 5 cm, find the perimeter of ∆APQ
Since AB and AC are the tangents from the same point A ∴AB=AC=5cm Similarly, BP=PX and XQ=QC Perimeter of \[\Delta APQ=AP+AQ+PQ\] \[=AP+AQ+(PX+XQ)\] \[=(AP+PX)+(AQ+XQ)\] \[=(AP+BP)+(AQ+QC)\]...
6. Rs. 9000 were divided equally among a certain number of persons. Had there been 20 more persons, each would have got Rs. 160 less. Find the original number of persons
Solution: Let’s consider the original number of people as ‘a’. Given that, $Rs.9000$ were divided equally among a certain number of persons. Had there been $20$ more persons, each would have got...
5. If the list price of a toy is reduced by Rs. 2, a person can buy 2 toys more for Rs. 360. Find the original price of the toy.
Solution: Let the original price of the toy be ‘x’. Given that, when the list price of a toy is reduced by $Rs.2$, the person can buy $2$ toys more for $Rs.360$. The number of toys he can buy at the...
3. A dealer sells an article for Rs. 24 and gains as much percent as the cost price of the article. Find the cost price of the article.
Solution: Let the cost price be assumed as Rs x. Given, the dealer sells an article for $Rs.24$ and gains as much percent as the cost price of the article. It’s given that he gains as much as the...
2. Some students planned a picnic. The budget for food was Rs. 480. But eight of these failed to go and thus the cost of food for each member increased by Rs. 10. How many students attended the picnic?
Solution: Let the number of students who planned the picnic be ‘x’. And given, budget for the food was $Rs.480$ So, cost of food for each member $= 480/x$ Also given, eight of these failed to go and...
1. A piece of cloth costs Rs. 35. If the piece were 4 m longer and each metre costs Rs. 1 less, the cost would remain unchanged. How long is the piece?
Solution: Let’s assume the length of the cloth to be ‘a’ meters. Given, piece of cloth costs $Rs.35$ and if the piece were $4m$ longer and each metre costs $Rs.1$ less, the cost remains unchanged....
A chord PQ of a circle is parallel to the tangent drawn at a point R of the circle. Prove that R bisects the arc PRQ.
Provided in question: Chord PQ is parallel to tangent at R.To prove: R bisects the arc PRQ. Proof: Since PQ || tangent at R. $\angle 1=\angle 2$ [alternate interior angles]$\angle 1=\angle 3$...
3. The general term of a sequence is given by an = -4n + 15. Is the sequence an A.P.? If so, find its 15th term and the common difference.
Solution: Given, ${{a}_{{{n}_{{}}}}}=-4n+15$ Now putting $n = 1, 2, 3, 4$ we get, ${{a}_{1}}=-4[1]+15=-4+15=11$ ${{a}_{2}}=-4[2]+15=-8+15=7$ ${{a}_{3}}=-4[3]+15=-12+15=3$ ...
Out of the two concentric circles, the radius of the outer circle is 5 cm and the chord AC of length 8 cm is a tangent to the inner circle. Find the radius of the inner circle.
Suppose C1 and C2 are two circles with the same center O. And AC is a chord touching C1 at the point D let’s join OD.So, $OD\bot AC$$AD=DC=4cm$ [perpendicular line OD...
2. Show that the sequence defined by an = 3n2 – 5 is not an A.P.
Solution: Given, ${{a}_{n}}=3{{n}_{2}}-5$ Now putting $n = 1, 2,3,4$ we get,...
If the quadrilateral sides touch the circle, prove that sum of pair of opposite sides is equal to the sum of other pair.
Let’s Consider a quadrilateral ABCD touching circle with the centre O at points E, F, G and H as we can see in figure. We know that, In a circle with two points outside of it, the tangents drawn...
Choose the correct answer from the given four options in the following questions: Which of the following is a quadratic equation?
(A) x2 + 2x + 1 = (4 – x)2 + 3 (B) –2x2 = (5 – x)(2x-(2/5)) (C) (k + 1) x2 + (3/2) x = 7, where k = –1 (D) x3 – x2 = (x – 1)3 (D) \[x3\text{ }\text{ }x2\text{ }=\text{ }\left( x\text{ }\text{ }1...
1. Show that the sequence defined by an = 5n – 7 is an A.P., find its common difference.
Solution: Given, $an = 5n – 7$ Now putting $n = 1, 2, 3, 4$ we get, ${{a}_{1}}=5[1]=5-7=-2$$$ ${{a}_{2=}}5[2]-7=10-7=3$ ${{a}_{3}}=5[3]-7=15-7=8$ ${{a}_{4}}=5[4]-7=20-7=13$ We can see that,...
7. An aero plane takes 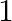 hour less for a journey of
hour less for a journey of 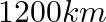 if its speed is increased by
if its speed is increased by 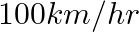 from its usual speed of the plane. Find its usual speed.
from its usual speed of the plane. Find its usual speed.
Solution: ...
A point P is 26 cm away from O of circle and the length PT of the tangent drawn from P to the circle is 10 cm. Find the radius of the circle.
Given, OP = $26cm$ PT = tangent length = $10cm$ To find: radius = OT =$?$ We know that, Radius and tangent are perpendicular at the point of contact, $\angle OTP={{90}^{\circ }}$ $$ So,...
Prove that one and only one out of n, n + 2 and n + 4 is divisible by 3, where n is any positive integer.
Solution: According to Euclid’s division Lemma, Let n be the positive integer And b equals to 3 Where q is the quotient and r is the remainder $n\text{ }=3q+r$, 0<r<3 implies remainders may be...
Show that the cube of a positive integer of the form 6q + r, q is an integer and r = 0, 1, 2, 3, 4, 5 is also of the form 6m + r.
Solution: Where q is an integer and r = 0, 1, 2, 3, 4, 5, then 6q + r is a positive integer. The positive integers are then of the form: 6q, 6q+1, 6q+2, 6q+3, 6q+4, and 6q+5. Taking cube on L.H.S...
Prove that if x and y are both odd positive integers, then x2 + y2 is even but not divisible by 4.
Solution: Let x and y be 2k + 1 and 2p + 1 the two odd positive numbers, respectively Therefore, ${{x}^{2}}~+\text{ }{{y}^{2}}~=\text{ }{{\left( 2k+\text{ }1 \right)}^{2}}~+{{\left( 2p\text{ }+1...
If n is an odd integer, then show that n2 – 1 is divisible by 8.
Solution: Any odd positive integer n can be written in the form 4q + 1 or 4q + 3 as we know. Now, according to the question, For $n~=4q~+1$, Therefore ${{n}^{2}}~-1\text{ }={{\left( 4q~+\text{ }1...
Show that the square of any odd integer is of the form 4q + 1, for some integer q.
Solution: Let b=4 and a be any odd integer. According to Euclid’s algorithm, For some integer $m\ge ~0,\text{ }a=4m+r$ And r = 0,1,2,3 as 0 ≤ r < 4. As a result we have, a = 4m or 4m +...
Show that the square of any positive integer cannot be of the form 6m + 2 or 6m + 5 for any integer m.
Solution: Let a be the positive integer. According to Euclid’s division algorithm, $a=6q+r$, where the value of 0 ≤ r < 6 ${{a}^{2}}~={{\left( 6q+r \right)}^{2}}~=36{{q}^{2}}~+\text{...
Show that the square of any positive integer cannot be of the form 5q + 2 or 5q + 3 for any integer q.
Solution: Let ‘a’ be the positive integer According to Euclid’s division lemma, $a=bm+r$ The value of b is 5 according to the question, $a=5m+r$ So, r= 0, 1, 2, 3, 4 For r = 0,$a=5m$. For r =...
Show that cube of any positive integer is of the form 4m, 4m + 1 or 4m + 3, for some integer m.
Solution: Let any positive integer be ‘a’ and the value b is 4. According to Euclid Division Lemma, $a=bq+r$, where, 0 ≤ r < b $a=3q+r$, where, 0 ≤ r < 4 The possible values of ‘r’ according...
Show that the square of any positive integer is either of the form 4q or 4q + 1 for some integer q.
Solution: According to Euclid’s division lemma, $~a=bq+r$ According to the question, For b = 4. $a=4k+r$, 0 < r < 4 For r = 0, we obtain, $a=4k$...
Find the zeroes of the following polynomials by factorisation method. 2×2 +(7/2)x +3/4
\[\begin{array}{*{35}{l}} 2x2\text{ }+\left( 7/2 \right)x\text{ }+3/4 \\ ~ \\ \end{array}\] The condition can likewise be composed as, \[8x2+14x+3\] Parting the...
Find the zeroes of the following polynomials by factorisation method. t3 – 2t2 – 15t
\[t3\text{ }\text{ }2t2\text{ }\text{ }15t\] Taking t normal, we get, \[t\text{ }\left( \text{ }t2\text{ }-\text{ }2t\text{ }-\text{ }15 \right)\] Parting the center term of the situation t2 - 2t -...
A doctor has prescribed a corrective lens of power +1.5 D. Find the focal length of the lens. Is the prescribed lens diverging or converging?
Answer- Expression for power of lens (P) = 1/f We are given that P = 1.5D Therefore, f = 1/1.5 f = 10/15 So, f = 0.66 m
Find the zeroes of the following polynomials by factorisation method. 5t2 + 12t + 7
\[5t2\text{ }+\text{ }12t\text{ }+\text{ }7\] Parting the center term, we get, \[5t2\text{ }+5t\text{ }+\text{ }7t\text{ }+\text{ }7\] Taking the normal factors out, we get, \[5t\text{ }\left( t+1...
Find the focal length of a lens of power -2.0 D. What type of lens is this?
Answer- According to the question, Power of the lens (P) is P = -2D And the expression for the power of the lens is P = 1/f So, we get => f = -1/2 f = -0.5 m As it has a negative focal length....
Find the zeroes of the following polynomials by factorisation method. 3×2 + 4x – 4
\[3x2\text{ }+\text{ }4x\text{ }\text{ }4\] Parting the center term, we get, \[3x2\text{ }+\text{ }6x\text{ }\text{ }2x\text{ }\text{ }4\] Taking the normal factors out, we get, \[3x\left( x+2...
Find the zeroes of the following polynomials by factorisation method.
1. 4x2 – 3x – 1 \[4x2\text{ }\text{ }3x\text{ }\text{ }1\] Parting the center term, we get, \[4x2-4x+1x-1\] Taking the normal factors out, we get, \[4x\left( x-1 \right)\text{ }+1\left(...
An object 5 cm is placed at a distance of 20 cm in front of a convex mirror of radius of curvature 30 cm. Find the position, nature and size of the image.
Answer- According to the question, object distance (u) is – 20 cm And object height (h) is 5 cm Radius of curvature (R) is 30 cm And we know that radius of curvature = 2 × Focal length Or, R = 2f We...
The magnification produced by a plane mirror is +1. What does this mean?
Answer- A virtual and erect image generated by a flat mirror is denoted by a positive sign. The size of the image is equal to the size of the object because the magnification is 1.
An object is placed at a distance of 10 cm from a convex mirror of focal length 15 cm. Find the position and nature of the image.
Answer- According to the focal length of convex mirror, (f) is +15 cm And the object distance (u) is – 10 cm Using the mirror formula, 1/f = 1/v - 1/u Or, 1/v = 1/f + 1/u = 1/15 - 1/(-10) v = 5/30 =...
A concave lens of focal length 15 cm forms an image 10 cm from the lens. How far is the object placed from the lens? Draw the ray diagram.
Answer- According to the question, focal length of concave lens (OF1) is f = – 15 cm Image distance (v) is – 10 cm Using the lens formula - 1/f = 1/v - 1/u Or, 1/v = 1/f + 1/u = - 1/(10) - 1/(-15)...
Given that √2 is a zero of the cubic polynomial 6×3 + √2 x2 – 10x – 4√2 , find its other two zeroes.
Given, √2 is one of the zero of the cubic polynomial. Then, at that point, (x-√2) is one of the factor of the given polynomial \[p\left( x \right)\text{ }=\text{ }6x{}^\text{3}+\surd...
Given that the zeroes of the cubic polynomial x3 – 6×2 + 3x + 10 are of the form a, a + b, a + 2b for some real numbers a and b, find the values of a and b as well as the zeroes of the given polynomial.
Considering that \[a,\text{ }a+b,\text{ }a+2b\] are foundations of given polynomial \[x{}^\text{3}-6x{}^\text{2}+3x+10\] Amount of the roots ⇒ \[a+2b+a+a+b\] = - coefficient of x²/coefficient of x³...
An object 5 cm in length is held 25 cm away from a converging lens of focal length 10 cm. Draw the ray diagram and find the position, size and the nature of the image formed.
Answer- According to the question, height of the Object h0 is 5 cm And the distance of the object from converging lens (u) is -25 cm Also the Focal length of a converging lens is f = 10 cm...
For each of the following, find a quadratic polynomial whose sum and product respectively of the zeroes are as given. Also find the zeroes of these polynomials by factorisation.
(iii) -2√3, -9 (iv) (-3/(2√5)), -½ (iii) Sum of the zeroes = – 2√3 Result of the zeroes = – 9 P(x) = x2 – (amount of the zeroes) + (result of the zeroes) Then, at that point, \[P\left( x...
For each of the following, find a quadratic polynomial whose sum and product respectively of the zeroes are as given. Also find the zeroes of these polynomials by factorisation.
(i) (–8/3), 4/3 (ii) 21/8, 5/16 (I) Sum of the zeroes = – 8/3 Result of the zeroes = 4/3 P(x) = x2 – (amount of the zeroes) + (result of the zeroes) Then, at that point, \[P\left( x \right)=\text{...
Answer the following and justify: (v) Can the quadratic polynomial x2 + kx + k have equal zeroes for some odd integer k > 1?
(v) A Quadratic Equation will have equivalent roots on the off chance that it fulfills the condition: \[b{}^\text{2}\text{ }\text{ }4ac\text{ }=\text{ }0\] Given condition is \[x{}^\text{2}\text{...
Answer the following and justify: (iii) If on division of a polynomial p (x) by a polynomial g (x), the quotient is zero, what is the relation between the degrees of p (x) and g (x)? (iv) If on division of a non-zero polynomial p (x) by a polynomial g (x), the remainder is zero, what is the relation between the degrees of p (x) and g (x)?
(iii) If on division of a polynomial p (x) by a polynomial g (x), the remainder is zero, what is the connection between the levels of p (x) and g (x)? We realize that, \[p\left( x \right)=\text{...
One-half of a convex lens is covered with a black paper. Will this lens produce a complete image of the object? Verify your answer experimentally. Explain your observations.
Answer- Yes, as indicated in the illustration, it will provide a comprehensive image of the object. The image of a distant object, such as a tree. on a screen when the lower part of the lens is...
Name the type of mirror used in the following situations.
(a) Headlights of a car (b) Side/rear-view mirror of a vehicle (c) Solar furnace Support your answer with reason. Answer- (a) Concave Because when the light source is placed at their major focus,...
Answer the following and justify:
(i) Can x2 – 1 be the quotient on division of x6 + 2x3 + x – 1 by a polynomial in x of degree 5? (ii) What will the quotient and remainder be on division of ax2 + bx + c by px3 + qx2 + rx + s, p ≠...
We wish to obtain an erect image of an object, using a concave mirror of focal length 15 cm. What should be the range of distance of the object from the mirror? What is the nature of the image? Is the image larger or smaller than the object? Draw a ray diagram to show the image formation in this case.
Answer- The object's distance from the mirror's pole ranges from 0 to 15 cm. The image's nature is virtual, upright, and larger than the thing.
Which of the following lenses would you prefer to use while reading small letters found in a dictionary?
(a) A convex lens of focal length 50 cm (b) A concave lens of focal length 50 cm (c) A convex lens of focal length 5 cm (d) A concave lens of focal length 5 cm Answer – The correct option is (c)....
Given that one of the zeroes of the cubic polynomial ax3 + bx2 + cx + d is zero, the product of the other two zeroes is
(A) (–c/a) (B) c/a (C) 0 (D) (–b/a) (B) (c/a) Clarification: As indicated by the inquiry, We have the polynomial, \[ax3\text{ }+\text{ }bx2\text{ }+\text{ }cx\text{ }+\text{ }d\] We realize that,...
The number of polynomials having zeroes as –2 and 5 is
(A) 1 (B) 2 (C) 3 (D) more than 3 (D) more than 3 Clarification: As per the inquiry, The zeroes of the polynomials = - 2 and 5 We realize that the polynomial is of the structure, \[p\left( x...
A spherical mirror and a thin spherical lens have a focal length of -15 cm. The mirror and the lens are likely to be
(a) both concave (b) both convex (c) the mirror is concave and the lens is convex (d) the mirror is convex, but the lens is concave Answer – (a) Both concave The focal length of a concave mirror and...
If the zeroes of the quadratic polynomial x2 + (a + 1) x + b are 2 and –3, then
(A) a = –7, b = –1 (B) a = 5, b = –1 (C) a = 2, b = – 6 (D) a = 0, b = – 6 (D) a = 2, b = – 6 Clarification: As per the inquiry, \[x{}^\text{2}\text{ }+\text{ }\left( a+1 \right)x\text{ }+\text{...
Where should an object be placed in front of a convex lens to get a real image of the size of the object?
(a) At the principal focus of the lens (b) At twice the focal length (c) At infinity (d) Between the optical centre of the lens and its principal focus. Answer – The correct option is (b). The...
The image formed by a concave mirror is observed to be virtual, erect and larger than the object. Where should be the position of the object?
(a) Between the principal focus and the centre of curvature (b) At the centre of curvature (c) Beyond the centre of curvature (d) Between the pole of the mirror and its principal focus. Answer- The...
Which one of the following materials cannot be used to make a lens?
(a) Water (b) Glass (c) Plastic (d) Clay Answer – The correct option is (d). Clay cannot be used to create a lens because light rays cannot flow through it.
A quadratic polynomial, whose zeroes are –3 and 4, is
(A) x2 – x + 12 (B) x2 + x + 12 (C) (x2/2)-(x/2)-6 (D) 2x2 + 2x –24 (C) \[\left( x2/2 \right)-\text{ }\left( x/2 \right)-\text{ }6\] Clarification: Amount of zeroes, \[\alpha +\text{ }\beta =\text{...
Find the power of a concave lens of focal length 2 m.
Answer- According to the question, focal length of concave lens (f) is 2 m Power of lens (P) = 1/f Power = 1/ (-2) Therefore, Power = -0.5D
A convex lens forms a real and inverted image of a needle at a distance of 50 cm from it. Where is the needle placed in front of the convex lens if the image is equal to the size of the object? Also, find the power of the lens.
Answer- Because the image is actual and the same size, it should be positioned at 2F. The image of the needle is assumed to be formed at a distance of 50 cm from the convex lens. As a result, the...
If one of the zeroes of the quadratic polynomial (k–1) x2 + k x + 1 is –3, then the value of k is
(A) 4/3 (B) -4/3 2/3 (D) -2/3 (A) 4/3 Clarification: As indicated by the inquiry, - 3 is one of the zeros of quadratic polynomial \[\left( k-1 \right)x2+kx+1\] Subbing - 3 in the given polynomial,...
5. The time taken by a person to cover 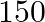 km was
km was 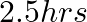 more than the time taken in the return journey. If he returned at the speed of
more than the time taken in the return journey. If he returned at the speed of 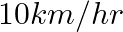 more than the speed of going, what was the speed per hour in each direction?
more than the speed of going, what was the speed per hour in each direction?
Solution: Let the ongoing speed of person be x km/hr, Then, the returning speed of the person is $= (x + 10) km/hr$ (from the question) Using, speed = distance/ time Time taken by the person in...
Define 1 dioptre of power of a lens.
Answer- The symbol D stands for dioptre, which is the SI unit of lens power. A dioptre is the power of a lens with a focal length of one metre.
4. A passenger train takes one hour less for a journey of 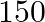 km if its speed is increased
km if its speed is increased 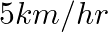 from its usual speed. Find the usual speed of the train.
from its usual speed. Find the usual speed of the train.
Solution: Let’s assume the usual speed of train as x km/hr Then, the increased speed of the train $= (x + 5) km/hr$ Using, speed = distance/ time Time taken by the train under usual speed to...
The refractive index of diamond is 2.42. What is the meaning of this statement?
Answer- Because diamond has a refractive index of 2.42, the speed of light in it is reduced by a factor of 2.42 when compared to the speed of light in air. To put it another way, the speed of light...
You are given kerosene, turpentine and water. In which of these does the light travel fastest? Use the information given in Table.
Material mediumRefractive indexMaterial mediumRefractiveindexAir1.0003Canada Balsam1.53Ice1.31––Water1.33Rock salt1.54Alcohol1.36––Kerosene1.44Carbon disulphide1.63Fusedquartz1.46Denseflint...
3. A fast train takes one hour less than a slow train for a journey of 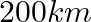 . If the speed of the slow train is
. If the speed of the slow train is 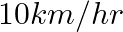 less than that of the fast train, find the speed of the two trains
less than that of the fast train, find the speed of the two trains
Solution: Let’s consider the speed of the fast train as x km/hr Then, the speed of the slow train will be $= (x -10) km/hr$ Using, speed = distance/ time Time taken by the fast train to cover $200...
2. A train, traveling at a uniform speed for 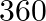 km, would have taken
km, would have taken 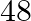 minutes less to travel the same distance if its speed were
minutes less to travel the same distance if its speed were 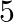 km/hr more. Find the original speed of the train.
km/hr more. Find the original speed of the train.
Solution: Let the original speed of train be x km/hr When increased by $5$, speed of the train $= (x + 5) km/hr$ Using, speed = distance/...
1. The speed of a boat in still water is 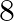 km/hr. It can go
km/hr. It can go 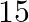 km upstream and
km upstream and 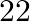 km downstream in
km downstream in 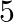 hours. Find the speed of the stream.
hours. Find the speed of the stream.
Solution: Let the speed of stream be x km/hr Given, speed of boat in still water is $8km/hr$. So, speed of downstream $= (8 + x) km/hr$ And, speed of upstream $= (8 – x) km/hr$ Using, speed =...
Find out, from Table, the medium having highest optical density. Also find the medium with lowest optical density.
MaterialmediumRefractive indexMaterial mediumRefractiveindexAir1.0003Canada Balsam1.53Ice1.31––Water1.33Rock salt1.54Alcohol1.36––Kerosene1.44Carbon disulphide1.63Fusedquartz1.46Denseflint...
Light enters from air to glass having refractive index 1.50. What is the speed of light in the glass? The speed of light in vacuum is 3 x 108 ms-1.
Answer- According to the question, speed of light in vacuum (c) is 3 × 108 m/s And refractive index of glass (ng) is 1.50 Expression for the speed of light in the glass (v) is given by - v = Speed...
A ray of light travelling in air enters obliquely into water. Does the light ray bends towards the normal or away from the normal? Why?
Answer- The light ray bends in the direction of normal. When a light ray passes through an optically rarer medium (low refractive index) and enters an optically denser medium (high refractive...
A concave mirror produces three times magnified (enlarged) real image of an object placed at 10 cm in front of it. Where is the image located?
Answer- Expression for magnification produced by a spherical mirror is - m = (height of image)/(height of object) Also, m = -(Image distance)/(object distance) So, we can write - (height of...
The radius of curvature of a spherical mirror is 32 cm. What is its focal length?
Answer- According to the question, radius of curvature (R) is 32 cm Expression for radius of curvature of the spherical mirror = 2 × Focal length (f) R = 2f Or, f= R/2 f= 32 / 2 f = 16 Therefore, 16...
Why do we prefer a convex mirror as a rear-view mirror in vehicles?
Answer- In cars and vehicles, a convex mirror is favoured as a rear-view mirror. This is because it provides a broader field of view, allowing the driver to see more of the traffic behind him....
Name the mirror that can give an erect and enlarged image of an object.
Answer- Concave Mirror is a mirror that may magnify and construct an object's image.
The radius of curvature of a spherical mirror is 20 cm. What is its focal length?
Answer- According to the question, radius of curvature (R) is 20 cm Expression for radius of curvature of the spherical mirror is = 2 × Focal length (f) So, R = 2f Or, f= R/2 = 20 / 2 f = 10...
A positive integer is of the form 3q + 1, q being a natural number. Can you write its square in any form other than 3m + 1, i.e., 3m or 3m + 2 for some integer m? Justify your answer.
Solution: No. Justification: Consider 3q + 1as the positive integer, where q is a natural number. (3q + 1)2 = 9q2 + 6q + 1 = 3(3q2 + 2q) + 1 = 3m + 1, (where m being an integer equals to 3q2 +...
Write whether the square of any positive integer can be of the form 3m + 2, where m is a natural number. Justify your answer.
Solution: No, the square of any positive integer cannot be of the form 3m + 2 where m is a natural number Justification: Applying the Euclid’s division lemma, The positive integer ‘a' can be written...
“The product of three consecutive positive integers is divisible by 6”. Is this statement true or false? Justify your answer.
Solution: Yes, the statement is true. Justification: Consider 2, 3, 4 as the three consecutive numbers \[\left( 2\text{ }\times \text{ }3\text{ }\times \text{ }4 \right)/6\text{ }=\text{ }24/6\text{...
“The product of two consecutive positive integers is divisible by 2”. Is this statement true or false? Give reasons.
Solution: Yes, the statement is true. Justification: Let ‘a’ and ‘a + 1’ be the two consecutive positive integers Applying the Euclid’s division lemma, We have, a = bq + r, where 0 ≤ r < b For...
Write whether every positive integer can be of the form 4q + 2, where q is an integer. Justify your answer.
Solution: No, every positive integer, where q is an integer cannot be of the form 4q + 2. Justification: All numbers in the form 4q + 2, where ‘q' is an integer, are even and not divisible by 4. For...
Define the principal focus of a concave mirror.
Answer- After reflecting from a concave mirror, light beams that are parallel to the principal axis converge at a certain location on the principal axis. The spot is the principal focus of the...
Choose the correct answer from the given four options in the following questions:
The largest number which divides 70 and 125, leaving remainders 5 and 8, respectively, is (A) 13 (B) 65 (C) 875 (D) 1750 Solution: Option(A) 13 Explanation: According to the question, Find the...
Choose the correct answer from the given four options in the following questions:
If the HCF of 65 and 117 is expressible in the form 65m – 117, then the value of m is: (A) 4 (B) 2 (C) 1 (D) 3 Solution: Option(B) 2 Explanation: Let's calculate the HCF of 65 and 117, 117 = 1×65 +...
Choose the correct answer from the given four options in the following questions:
n2 – 1 is divisible by 8, if n is: (A) an integer (B) a natural number (C) an odd integer (D) an even integer Solution: Option(C) an odd integer Explanation: Let x = n2 – 1 In the equation...
Choose the correct answer from the given four options in the following questions:
For some integer q, every odd integer is of the form: (A) q (B) q +1 (C) 2g (D) 2q +1 Solution: Option(D) i.e. 2q+1 Explanation: Those integers which are not divisible by 2 are odd integers. As a...
Choose the correct answer from the given four options in the following questions:
For some integer m, every even integer is of the form: (A) m (B) m +1 (C) 2m (D) 2m+1 Solution: Option(C) 2m Explanation: Those integers which are divisible by 2 are even integers. As a result, it...
A farmer connects a pipe of internal diameter 20 cm from a canal into a cylindrical tank in her field, which is 10 m in diameter and 2 m deep. If water flows through the pipe at the rate of 3 km/h, in how much time will the tank be filled?
Think about the accompanying graph Volume of water that streams in t minutes from pipe \[=\text{ }t\times 0.5\pi \text{ }m^3\] Volume of water that streams in t minutes from pipe = \[t\times 0.5\pi...
In a garden pea’s flower, if the megaspore mother cell forms megaspores without undergoing meiosis and if one of the megaspores develops into an embryo sac, its nuclei would be ?
a. Haploid dominantly b. With varying ploidy. c. A few haploid and a diploid d. Diploid Solution: Option (d) is the answer.
2. Prove that the product of two consecutive positive integers is divisible by 2.
Solution: Real numbers are simply the combination of rational and irrational numbers, in the number system. In general, all the arithmetic operations can be performed on these numbers and they...
1. Draw an ogive by less than the method for the following data:
No. of rooms12345678910No. of houses49222824128652 Solution: No. of roomsNo. of housesCumulative FrequencyLess than or equal to 144Less than or equal to 2913Less than or equal to 32235Less than or...
6. The following is the distribution of height of students of a certain class in a city:
Height (in cm):160 – 162163 – 165166 – 168169 – 171172 – 174No of students:1511814212718 Find the average height of maximum number of students. Solution: Statistics is the discipline that...
1. In a Δ ABC, AD is the bisector of ∠ A, meeting side BC at D. (vii) if ![Rendered by QuickLaTeX.com \[\mathbf{AB}\text{ }=\text{ }\mathbf{5}.\mathbf{6}\text{ }\mathbf{cm},\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-5bda87937897bc7402395a0e7497aa6b_l3.png)
![Rendered by QuickLaTeX.com \[\mathbf{BC}\text{ }=\text{ }\mathbf{6}\text{ }\mathbf{cm},\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-cc05f2b9f3ba514ca9c9b35898d5010a_l3.png)
and ![Rendered by QuickLaTeX.com \[\mathbf{BD}\text{ }=\text{ }\mathbf{3}.\mathbf{2}\text{ }\mathbf{cm},\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-2cb291882d238e8010337f40ada99f74_l3.png)
find AC. (viii) if ![Rendered by QuickLaTeX.com \[\mathbf{AB}\text{ }=\text{ }\mathbf{10}\text{ }\mathbf{cm},\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-49cecb59e896652d21a82c8db7df1c60_l3.png)
![Rendered by QuickLaTeX.com \[\mathbf{AC}\text{ }=\text{ }\mathbf{6}\text{ }\mathbf{cm},\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-8fcf95066978bb3a5c81e31da13743b6_l3.png)
and ![Rendered by QuickLaTeX.com \[\mathbf{BC}\text{ }=\text{ }\mathbf{12}\text{ }\mathbf{cm},\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-2442b612d2d4da073e2ebd36b6adccf8_l3.png)
find BD and DC.
Solution: Given: Δ ABC and AD bisects ∠A, meeting side BC at D. \[AB\text{ }=\text{ }5.6\text{ }cm,\] \[BC\text{ }=\text{ }6\text{ }cm,\] and \[BD\text{ }=\text{ }3.2\text{ }cm\]....
14. The lengths of the diagonals of a rhombus is ![Rendered by QuickLaTeX.com \[\mathbf{24cm}\text{ }\mathbf{and}\text{ }\mathbf{10cm}.\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-c85c0e710149fb9633b94c8c2abffd5a_l3.png)
Find each side of the rhombus.
Solution: Let ABCD be a rhombus and AC and BD be the diagonals of ABCD. So, AC = \[24cm\text{ }and\text{ }BD\text{ }=\text{ }10cm\] \[\] We know that diagonals of a rhombus bisect each other at...
13. In a ∆ABC, AB = BC = CA = ![Rendered by QuickLaTeX.com \[\mathbf{2a}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-dc21b199c4adb0b51f7839a0d5967962_l3.png)
and AD ⊥ BC. Prove that
(i) AD = a\[\surd \mathbf{3}\] (ii) Area (∆ABC) = \[\surd \mathbf{3}\text{ }{{\mathbf{a}}^{\mathbf{2}}}\] Solution: (i) In ∆ABD and ∆ACD, we have∠ADB = ∠ADC = \[{{90}^{o}}\]AB = AC [Given]AD =...
12. In an isosceles triangle ABC, if AB = AC = ![Rendered by QuickLaTeX.com \[\mathbf{13cm}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-0a3890f5045c3fc67ed8d0dc3fee80ac_l3.png)
and the altitude from A on BC is
\[\mathbf{5cm},\]find BC. Solution: Given, An isosceles triangle ABC, AB = AC = \[13cm,\text{ }AD\text{ }=\text{ }5cm\] Required to find: BC In ∆ ADB, by using Pythagoras theorem, we have...
11. ABCD is a square. F is the mid-point of AB. BE is one third of BC. If the area of ∆ FBE = ![Rendered by QuickLaTeX.com \[\mathbf{108c}{{\mathbf{m}}^{\mathbf{2}}}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-aea3dbba23caac08c8a64b556bd738bb_l3.png)
, find the length of AC.
Solution: Given, ABCD is a square. And, F is the mid-point of AB. BE is one third of BC. Area of ∆ FBE = \[108c{{m}^{2}}\] Required to find: length of AC Let’s assume the sides of the square to be...
10. A triangle has sides ![Rendered by QuickLaTeX.com \[\mathbf{5}\text{ }\mathbf{cm},\text{ }\mathbf{12}\text{ }\mathbf{cm}\text{ }\mathbf{and}\text{ }\mathbf{13}\text{ }\mathbf{cm}.\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-55595a46a936f27b67a56d78fd6d9f6b_l3.png)
Find the length to one decimal place, of the perpendicular from the opposite vertex to the side whose length is ![Rendered by QuickLaTeX.com \[\mathbf{13}\text{ }\mathbf{cm}.\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-dd9682929f90290113c48dab79d05fcc_l3.png)
Solution: From the fig. \[AB\text{ }=\text{ }5cm,\text{ }BC\text{ }=\text{ }12\text{ }cm\text{ }and\text{ }AC\text{ }=\text{ }13\text{ }cm.\] Then, \[A{{C}^{2}}~=\text{ }A{{B}^{2}}~+\text{...
9. Using Pythagoras theorem determine the length of AD in terms of b and c shown in Fig ![Rendered by QuickLaTeX.com \[.\text{ }\mathbf{4}.\mathbf{219}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-e0e6c8ae5a8e273c9a6be45b04989009_l3.png)
Solution: We have, In ∆BAC, by Pythagoras theorem, we have \[\begin{array}{*{35}{l}} B{{C}^{2}}~=\text{ }A{{B}^{2}}~+\text{ }A{{C}^{2}} \\ \Rightarrow...
8. Two poles of height ![Rendered by QuickLaTeX.com \[\mathbf{9}\text{ }\mathbf{in}\text{ }\mathbf{and}\text{ }\mathbf{14}\text{ }\mathbf{m}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-a5efcdbe8ed132a615044b2b9e1efbd4_l3.png)
stand on a plane ground. If the distance between their feet is ![Rendered by QuickLaTeX.com \[\mathbf{12}\text{ }\mathbf{m},\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-18666dfb8dc6e2f5280fce139ea621e6_l3.png)
find the distance between their tops.
Solution: Comparing with the figure, it’s given that AC = \[14\text{ }m,\text{ }DC\text{ }=\text{ }12m\text{ }and\text{ }ED\text{ }=\text{ }BC\text{ }=\text{ }9\text{ }m\] Construction: Draw...
7. The foot of a ladder is ![Rendered by QuickLaTeX.com \[\mathbf{6}\text{ }\mathbf{m}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-0a07f6483f265e6e47c316121ac8cca7_l3.png)
away from a wall and its top reaches a window ![Rendered by QuickLaTeX.com \[\mathbf{8}\text{ }\mathbf{m}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-f5dd6c4c521acc9e7e7137b3036d18fb_l3.png)
above the ground. If the ladder is shifted in such a way that its foot is ![Rendered by QuickLaTeX.com \[\mathbf{8}\text{ }\mathbf{m}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-f5dd6c4c521acc9e7e7137b3036d18fb_l3.png)
away from the wall, to what height does its tip reach?
Solution: Let’s assume the length of ladder to be, AD = BE = x m So, in ∆ACD, by Pythagoras theorem We have, \[\begin{array}{*{35}{l}} ...
6. In an isosceles triangle ABC, AB = ![Rendered by QuickLaTeX.com \[\mathbf{AC}\text{ }=\text{ }\mathbf{25}\text{ }\mathbf{cm},\text{ }\mathbf{BC}\text{ }=\text{ }\mathbf{14}\text{ }\mathbf{cm}.\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-49a79c647484ca1f07eb3412c6395529_l3.png)
Calculate the altitude from A on BC.
Solution: Given, ∆ABC, AB = AC = \[25\text{ }cm\text{ }and\text{ }BC\text{ }=\text{ }14.\] \[\] In ∆ABD and ∆ACD, we see...
5. Two poles of heights ![Rendered by QuickLaTeX.com \[\mathbf{6}\text{ }\mathbf{m}\text{ }\mathbf{and}\text{ }\mathbf{11}\text{ }\mathbf{m}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-ce51d1c0d04bc918a23933dbe2116e9a_l3.png)
stand on a plane ground. If the distance between their feet is ![Rendered by QuickLaTeX.com \[\mathbf{12}\text{ }\mathbf{m}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-5b1a778c62eb81bbb683b987a69b68d9_l3.png)
, find the distance between their tops.
Solution: Let CD and AB be the poles of height \[11m\text{ }and\text{ }6m.\] Then, its seen that CP = \[11\text{ }\text{ }6\text{ }=\text{ }5m.\] From the figure, AP should be \[12m\] (given) In...
4. A ladder ![Rendered by QuickLaTeX.com \[\mathbf{17}\text{ }\mathbf{m}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-a8857d36c65c9db76f01d8b00e922493_l3.png)
long reaches a window of a building ![Rendered by QuickLaTeX.com \[\mathbf{15}\text{ }\mathbf{m}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-f363f724150e591edf9182f4311ab3e8_l3.png)
above the ground. Find the distance of the foot of the ladder from the building.
Solution: In ∆ABC, by Pythagoras theorem \[\begin{array}{*{35}{l}} A{{B}^{2}}~+\text{ }B{{C}^{2}}~=\text{ }A{{C}^{2}} \\ \Rightarrow {{15}^{2}}~+\text{...
3. A man goes ![Rendered by QuickLaTeX.com \[\mathbf{15}\text{ }\mathbf{metres}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-712e26540491c89b51fc3bb96dfabd2f_l3.png)
due west and then ![Rendered by QuickLaTeX.com \[\mathbf{8}\text{ }\mathbf{metres}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-9d6db488112b88ddeb15d838748fedad_l3.png)
due north. How far is he from the starting point?
Solution: ...
2. The sides of certain triangles are given below. Determine which of them are right triangles.
\[\begin{array}{*{35}{l}} \left( \mathbf{i} \right)\text{ }\mathbf{a}\text{ }=\text{ }\mathbf{7}\text{ }\mathbf{cm},\text{ }\mathbf{b}\text{ }=\text{ }\mathbf{24}\text{...
1. If the sides of a triangle are ![Rendered by QuickLaTeX.com \[\mathbf{3}\text{ }\mathbf{cm},\text{ }\mathbf{4}\text{ }\mathbf{cm},\text{ }\mathbf{and}\text{ }\mathbf{6}\text{ }\mathbf{cm}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-4e7f08d95ac5afd7a7bf17c308701538_l3.png)
long, determine whether the triangle is a right-angled triangle.
Solution: We have, Sides of triangle as \[\begin{array}{*{35}{l}} AB\text{ }=\text{ }3\text{ }cm \\ BC\text{ }=\text{ }4\text{ }cm \\ AC\text{...
1. Calculate the mean for the given distribution:
$x:$$5$$6$$7$$8$$9$$f:$$4$$8$$14$$11$$3$ Solution: $x$$f$$fx$$5$$4$$20$$6$$8$$48$$7$$14$$98$$8$$11$$88$$9$$3$$27$$N=40$$\sum{fx=281}$ Mean $=\sum{fx/n=281/40}$ $\therefore $ Mean...
Usually, deciduous plants shed their leaves during summer or in autumn. This process of shedding of leaves is called abscission. Which is the anatomical mechanism is involved in the abscission of leaves?
Solution: i. Structural: In deciduous trees, separation zone is formed at the base of the petiole. It is composed of a top layer(distal) and a bottom (proximal)layer. The cells in the top layer have...
4. Can two numbers have ![Rendered by QuickLaTeX.com \[\mathbf{16}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-ec8d9d2d731b4ca460e57ad46a988282_l3.png)
as their HCF and ![Rendered by QuickLaTeX.com \[\mathbf{380}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-cb340828e6c6101fbde249e54a9cd1cb_l3.png)
as theirLCM? Give reason.
Solution: On dividing \[380\]by \[16\]we get,\[23\]as the quotient and\[12\]as the remainder. Now, since the LCM is not exactly divisible by the HCF its can be said that two numbers cannot have...
2. Find the LCM and HCF of the following integers by applying the prime factorization method:(v) ![Rendered by QuickLaTeX.com \[\mathbf{84},\text{ }\mathbf{90}\text{ }\mathbf{and}\text{ }\mathbf{120}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-ccfecca6ebaa7e44c50bda4ca8c75939_l3.png)
(vi] ![Rendered by QuickLaTeX.com \[\mathbf{24},\text{ }\mathbf{15}\text{ }\mathbf{and}\text{ }\mathbf{36}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-94f859fd8a71046e251dab20e2d08dcb_l3.png)
Solution: First, Find the prime factors of the given integers: \[84,\text{ }90\text{ }and\text{ }120\] For, \[\begin{array}{*{35}{l}} ~84\text{...
2. Find the LCM and HCF of the following integers by applying the prime factorization method:(iii) ![Rendered by QuickLaTeX.com \[\mathbf{8},\text{ }\mathbf{9}\text{ }\mathbf{and}\text{ }\mathbf{25}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-78297b7f285a41777161d421fedfd300_l3.png)
(iv) ![Rendered by QuickLaTeX.com \[\mathbf{40},\text{ }\mathbf{36}\text{ }\mathbf{and}\text{ }\mathbf{126}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-17883cf51f0a9421203bd7e5576dc901_l3.png)
Solution: First, find the prime factors of the given integers: 8, 9 and 25 For, \[8\text{ }=\text{ }2\text{ }\times...
2. Find the LCM and HCF of the following integers by applying the prime factorization method:
(i) 12, 15 and 21(ii) \[\mathbf{17},\text{ }\mathbf{23}\text{ }\mathbf{and}\text{ }\mathbf{29}\] Solution: First, find the prime factors of the given integers: \[12,\text{ }15\text{ }and\text{...
1. Find the LCM and HCF of the following pairs of integers and verify that LCM × HCF = Product of the integers:
(i) \[\mathbf{26}\text{ and }\mathbf{91}\](ii) \[\mathbf{510}\text{ and }\mathbf{92}\] Solution: Given integers are\[\mathbf{26}\text{ and...
9. Prove that ![Rendered by QuickLaTeX.com \[\surd 5\text{ }+\text{ }\surd 3~\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-0efb8e4ff13d751cc9c194f8bc9b1b82_l3.png)
is irrational.
Solution: Let’s assume on the contrary that \[\surd 5\text{ }+\text{ }\surd 3\] is a rational number. Then, there exist co prime positive integers a and b such that\[\surd 5\text{ }+\text{ }\surd...
8. Prove that ![Rendered by QuickLaTeX.com \[\mathbf{2}\text{ }-\text{ }\mathbf{3}\surd \mathbf{5}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-492fc7220996be5495f8ab5970d52502_l3.png)
is an irrational number.
Solution: Let’s assume on the contrary that \[2\text{ }\text{ }3\surd 5\]is a rational number. Then, there exist co prime positive integers a and b such that \[2\text{ }\text{ }3\surd 5\text{...
6. Show that ![Rendered by QuickLaTeX.com \[\mathbf{5}\text{ }-\text{ }\mathbf{2}\surd \mathbf{3}~\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-4fe99f6574a18dae472d801d66331d39_l3.png)
is an irrational number.
Solution: Let’s assume on the contrary that \[5\text{ }\text{ }2\surd 3\] is a rational number. Then, there exist co prime positive integers a and b such that \[5\text{ }\text{ }2\surd 3\text{...
5. Prove that ![Rendered by QuickLaTeX.com \[\mathbf{4}\text{ }-\text{ }\mathbf{5}\surd \mathbf{2}~\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-728fc3aae21f5413024bcf441fffe566_l3.png)
is an irrational number.
Solution: Let’s assume on the contrary that \[4\text{ }\text{ }5\surd 2\] is a rational number. Then, there exist co prime positive integers a and b such that \[4\text{ }\text{ }5\surd 2\text{...
4. Show that ![Rendered by QuickLaTeX.com \[\mathbf{3}\text{ }+\text{ }\surd \mathbf{2}~\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-fc1c82b2f160b7a1dc310519dbde254a_l3.png)
is an irrational number
Solution: Let’s assume on the contrary that \[3\text{ }+\text{ }\surd 2\] is a rational number. Then, there exist co prime positive integers a and b such that \[3\text{ }+\text{ }\surd 2=\text{...
3. Show that ![Rendered by QuickLaTeX.com \[\mathbf{2}\text{ }-\text{ }\surd \mathbf{3}~\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-d0a39d7c5e26f9ab889ba6084fa2705f_l3.png)
is an irrational number.
Solution: Let’s assume on the contrary that \[2\text{ }\text{ }\surd 3\] is a rational number. Then, there exist co prime positive integers a and b such that\[2\text{ }\text{ }\surd 3=\text{ }a/b\]...
2. Prove that the following numbers are irrationals.(iii) ![Rendered by QuickLaTeX.com \[\mathbf{4}\text{ }+\text{ }\surd \mathbf{2}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-e72e18bc9159e65a933a5182ce232b1e_l3.png)
(iv) ![Rendered by QuickLaTeX.com \[\mathbf{5}\surd \mathbf{2}~~~~~~~~~\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-f66067211a2d0fa3d76358415a69dc68_l3.png)
Solution: Let’s assume on the contrary that \[4\text{ }+\text{ }\surd 2\] is a rational number. Then, there exist co prime positive integers a and b such that \[4\text{ }+\text{ }\surd 2\text{...
Solve each of the following systems of equations by the method of cross-multiplication:
\[\mathbf{13}.\]\[~\mathbf{x}/\mathbf{a}\text{ }+\text{ }\mathbf{y}/\mathbf{b}\text{ }=\text{ }\mathbf{a}\text{ }+\text{ }\mathbf{b}\] \[\mathbf{x}/{{\mathbf{a}}^{\mathbf{2}~}}+\text{...
1. Show that the following numbers are irrational.(iii) ![Rendered by QuickLaTeX.com \[\mathbf{6}\text{ }+\text{ }\surd \mathbf{2}\]](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-3751ac6d34393366c041894ce911cbaa_l3.png)
Solution: Let’s assume on the contrary that \[6+\surd 2\] is a rational number. Then, there exist co prime positive integers a and b such that \[6\text{ }+\text{ }\surd 2\text{ }=\text{ }a/b\]...
In the double displacement reaction between aqueous potassium iodide and aqueous lead nitrate, a yellow precipitate of lead iodide is formed. While performing the activity if lead nitrate is not available, which of the following can be used in place of lead nitrate?
(a) Lead sulphate (insoluble)
(b) Lead acetate
(c) Ammonium nitrate
(d) Potassium sulphate
Solution: The correct response is (b) lead acetate. Explanation: Because we need a compound that contains lead in order to obtain lead iodide, ammonium nitrate and potassium sulphate are ruled out....
Electrolysis of water is a decomposition reaction. The mole ratio of hydrogen and oxygen gases liberated during electrolysis of water is
(a) 1:1
(b) 2:1
(c) 4:1
(d) 1:2
Answer: option b Water contains two moles of hydrogen and one mole of water in a single mole. Because of this, the mole ratio of hydrogen to oxygen is 2:1.
Barium chloride on reacting with ammonium sulphate forms barium sulphate and ammonium chloride. Which of the following correctly represents the type of the reaction involved?
(i) Displacement reaction
(ii) Precipitation reaction
(iii) Combination reaction
(iv) Double displacement reaction
(a) (i) only
(b) (ii) only
(c) (iv) only
(d) (ii) and (iv)
Solution: Answer is option d) The elements ammonium and barium are being displaced from their respective salts, according to the data. As a result, we have a double displacement reaction. Because of...
Which among the following statement(s) is (are) true? Exposure of silver chloride to sunlight for a long duration turns grey due to
(i) the formation of silver by decomposition of silver chloride
(ii) sublimation of silver chloride
(iii) decomposition of chlorine gas from silver chloride
(iv) oxidation of silver chloride
(a) (i) only
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (iv) only
Solution: Answer is option a) Silver halides, particularly silver chloride, decompose in the presence of sunlight, resulting in the formation of silver metal and a halogen gas (silver) (chlorine or...
Solid calcium oxide reacts vigorously with water to form calcium hydroxide accompanied by the liberation of heat. This process is called slaking of lime. Calcium hydroxide dissolves in water to form its solution called lime water. Which among the following is (are) true about slaking of lime and the solution formed?
(i) It is an endothermic reaction
(ii) It is an exothermic reaction
(iii) The pH of the resulting solution will be more than seven
(iv) The pH of the resulting solution will be less than seven
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (iii) and (iv)
Solution: The answer is (b) (ii) and (iii) In the presence of vigorous water reaction, solid calcium oxide transforms into calcium hydroxide, which is accompanied by the production of heat. This...
Which among the following is(are) double displacement reaction(s)?
(i) Pb + CuCl2 → PbCl2 + Cu
(ii) Na2 SO4 + BaCl2 → BaSO4 + 2NaCl
(iii) C + O2 → CO2
(iv) CH4 + 2O2 → CO2 + 2H2O
(a) (i) and (iv)
(b) (ii) only
(c) (i) and (ii)
(d) (iii) and (iv)
Solution: The answer is (b) (ii) only Sodium and barium are both displaced from their respective salts in this reaction, which is referred to as a double displacement reaction.
A dilute ferrous sulphate solution was gradually added to the beaker containing acidified permanganate solution. The light purple colour of the solution fades and finally disappears. Which of the following is the correct explanation for the observation?
(a) KMnO4 is an oxidising agent, it oxidises FeSO4
(b) FeSO4 acts as an oxidising agent and oxidises KMnO4
(c) The colour disappears due to dilution; no reaction is involved
(d) KMnO4 is an unstable compound and decomposes in the presence of FeSO4 to a colourless compound.
Solution: Answer is (a) KMnO4 is an oxidising agent, it oxidises FeSO4 Potassium permanganate (KMnO4) is used as an oxidising agent during this reaction. This is due to the presence of KMnO4, which...
Three beakers labelled as A, B and C each containing 25 mL of water were taken. A small amount of NaOH, anhydrous CuSO4 and NaCl were added to the beakers A, B and C respectively. It was observed that there was an increase in the temperature of the solutions contained in beakers A and B, whereas, in the case of beaker C, the temperature of the solution falls. Which one of the following statements(s) is(are) correct?
(i) In beakers A and B, the exothermic process has occurred.
(ii) In beakers A and B, the endothermic process has occurred.
(iii) In beaker C exothermic process has occurred.
(iv) In beaker C endothermic process has occurred.
(a) (i) only
(b) (ii) only
(c) (i) and (iv)
(d) (ii) and (iii)
Answer: Answer is (c) (i) and (iv) Exothermic processes result in the release of enormous amounts of heat – as a result, when water reacts with quick lime and when acid reacts with water, heat...
Which of the following are exothermic processes?
(i) The reaction of water with quick lime
(ii) Dilution of an acid
(iii) Evaporation of water
(iv) Sublimation of camphor (crystals)
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (iii) and (iv)
Answer: Option a) Exothermic processes result in the release of enormous amounts of heat – as a result when water reacts with quick lime and when an acid reacts with water, heat energy is...
Which of the following statements about the given reaction are correct? 3Fe(s) + 4H2O(g) → Fe3O4 (s) + 4H2 (g)
1. Iron metal is getting oxidised
2. Water is getting reduced
3. Water is acting as a reducing agent
4. Water is acting as an oxidising agent
(a) (i), (ii) and (iii)
(b) (iii) and (iv)
(c) (i), (ii) and (iv)
(d) (ii) and (iv)
Answer: Option c) In this reaction, oxygen reacts with water to form oxidised water. Because oxygen is being removed from water, the water's oxygen content is being reduced. Water is a source of...
The following reaction is an example of a:4NH3 (g) + 5O2 (g) → 4NO(g) + 6H2O(g)
(i) displacement reaction
(ii) combination reaction
(iii) redox reaction
(iv) neutralisation reaction
(a) (i) and (iv)
(b) (ii) and (iii)
(c) (i) and (iii)
(d) (iii) and (iv)
Solution: Answer is (c) (i) and (iii) The reaction described here is a combination of displacement and redox reactions. The displacement reaction occurs when oxygen displaces hydrogen in ammonia,...
Which of the following is not a physical change?
(a) Boiling of water to give water vapour
(b) Melting of ice to give water
(c) Dissolution of salt in water
(d) Combustion of Liquefied Petroleum Gas (LPG)
Answer: Answer is (d) Combustion of Liquefied Petroleum Gas (LPG) Because a new compound is formed after burning, combustion is always accompanied by a chemical change, which is irreversible in...
Write the balanced chemical equations for the following reactions and identify the type of reaction in each case.
(c) Ethanol is warmed with ethanoic acid to form ethyl acetate in the presence of concentrated 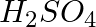
(d) Ethene is burnt in the presence of oxygen to form carbon dioxide, water and releases heat and light.
Answer: (c) $C_2 H_5 OH ( aq )+ CH_3 COOH (l) \longrightarrow CH_3 COO C_2 H_5( aq )+ H_2 O ( t )$It is a neutralisation reaction and double displacement reaction.(d) $C_{2} H_{4}( g )+3 O_{2}(g)...
Write the balanced chemical equations for the following reactions and identify the type of reaction in each case.
(a) Nitrogen gas is treated with hydrogen gas in the presence of a catalyst at 773K to form ammonia gas.
(b) Sodium hydroxide solution is treated with acetic acid to form sodium acetate and water.
Answer: (a) $N {2}( g )+3 H {2}( g )=\frac{\text { catalyst }}{773 K } 2 NH {2}( g )$ It is a combination reaction. (b) $NaOH ( aq )+ CH _{3} COOH ( aq ) \longrightarrow CH_3 COONa ( aq )+ H_2 O...
Write the balanced chemical equations for the following reactions and identify the type of reaction in each case.
(c) Chlorine gas is passed in an aqueous potassium iodide solution to form potassium chloride solution and solid iodine.
(d) Ethanol is burnt in air to form carbon dioxide, water and releases heat.
Answer: c) Aqueous potassium iodide solution is passed through a chlorine gas stream, resulting in the formation of potassium chloride solution and solid iodine.This is a reaction with a single...
Write the balanced chemical equations for the following reactions and identify the type of reaction in each case.
(a) Thermite reaction, iron (III) oxide reacts with aluminium and gives molten iron and aluminium oxide.
(b) Magnesium ribbon is burnt in an atmosphere of nitrogen gas to form solid magnesium nitride.
Answer: a) Iron (III) oxide reacts with aluminium to form molten iron and aluminium oxide in the first step of the Thermit reaction. This is a reaction with a single displacement. Fe2O3 + 2Al →...
Complete the missing components/variables given as x and y in the following reactions
(c) 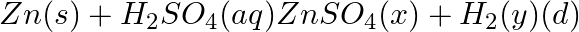 CaCO_3 (s) →xCaO(s) + CO_2 (g)$
CaCO_3 (s) →xCaO(s) + CO_2 (g)$
Solution: (c) Zn(s) + H2 SO4 (aq) → ZnSO4 (aq) + H2 (g) (d) CaCO3 (s) →heat→ CaO(s) + CO2 (g) x-(aq) y(g)x is heat
Complete the missing components/variables given as x and y in the following reactions
(a) 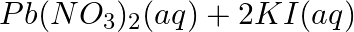 →
→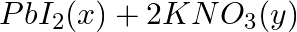
(b) 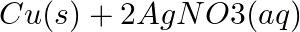 →
→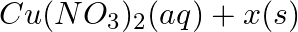
Answer: (a) Pb(NO3 ) 2 (aq) + 2KI(aq) → PbI2 (s) + 2KNO3 (aq) (b) Cu(s) + 2Ag NO3 (aq) → Cu(NO3 ) 2 (aq) + 2Ag(s) x(s), y (aq)x is 2Ag
Which among the following changes are exothermic or endothermic in nature?
(a) Decomposition of ferrous sulfate
(b) Dilution of sulphuric acid
(d) Dissolution of ammonium chloride in water
Answer:An exothermic process is one that generates heat as a byproduct. A portion of this heat is transferred to the surrounding environment. Heat must be supplied to the system from the surrounding...
Identify the reducing agent in the following reactions
(a) 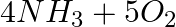

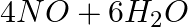
(b) 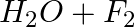

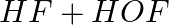
Answer: In a redox chemical reaction, a reducing agent is an element or compound that "donates" or "loses" an electron to an electron recipient as part of the reaction. NH3-AmmoniaH2O – Water
Identify the reducing agent in the following reactions
(c) 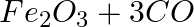 →
→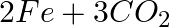
(d) 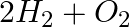 →
→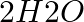
Answer: In a redox chemical reaction, a reducing agent is an element or compound that "donates" or "loses" an electron to an electron recipient as part of the reaction. CO-Carbon momnoxide$H_2$-...
Identify the oxidizing agent (oxidant) in the following reactions
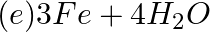 →
→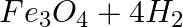
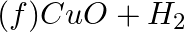 →
→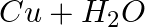
Answer: According to chemistry, an oxidising agent, or oxidising agent, is a substance that has the ability to oxidise other substances, or to accept electrons from them, in other words, it is an...
Identify the oxidizing agent (oxidant) in the following reactions
(c) 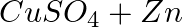 →
→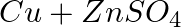
(d) 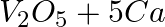 →
→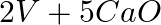
Answer:According to chemistry, an oxidising agent, or oxidising agent, is a substance that has the ability to oxidise other substances, or to accept electrons from them, in other words, it is an...
Identify the oxidizing agent (oxidant) in the following reactions
(a) 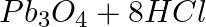 →
→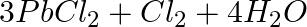
(b) 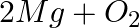 →
→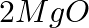
Answer:According to chemistry, an oxidising agent, or oxidising agent, is a substance that has the ability to oxidise other substances, or to accept electrons from them, in other words, it is an...
Write the balanced chemical equations for the following reactions (c) Copper sulfate on treatment with potassium iodide precipitates cuprous iodide (Cu2 I2 ), liberates iodine gas, and also forms potassium sulfate.
Solution: (c) 2CuSO4+4Kl →$2K_2SO_4+Cu_2I_2+I_2$
Write the balanced chemical equations for the following reactions
(a) Sodium carbonate on reaction with hydrochloric acid in equal molar concentrations gives sodium chloride and sodium hydrogen carbonate.
(b) Sodium hydrogen carbonate on reaction with hydrochloric acid gives sodium chloride, water and liberates carbon dioxide.
Answer: (a) Na2CO3 + HCl → NaCl + NaHCO3 (b) NaHCO3 + HCl → NaCl + H2O + CO2
In a solution of potassium chloride when mixed with silver nitrate solution, an insoluble white substance is formed. Write the chemical reaction involved and also mention the type of chemical reaction?
Solution: Double displacement reactions are chemical reactions in which one component of each of the reacting molecules is exchanged in order to form the products, as defined by the American...
Ferrous sulfate decomposes with the evolution of a gas having a characteristic odor of burning sulfur. Write the chemical reaction involved and identify the type of reaction.
Answer: When heated, ferrous sulfate decomposes into ferric oxide, sulphur dioxide, and sulphur trioxide, which are all toxic. Using this balanced equation, we can express the thermal decomposition...
Why do fireflies glow at night?
Answer: During the course of the night, fireflies produce a chemical reaction within their bodies that allows them to glow. In the presence of an enzyme known as luciferase, oxygen reacts with...
Grapes hanging on the plant do not ferment but after being plucked from the plant can be fermented. Under what conditions do these grapes ferment? Is it a chemical or a physical change?
Answer: The defense mechanism of plants prevents the fermentation of grapes on the plant. When grapes are plucked from the vine, they react with yeast to produce fermentation, which is the final...
During the reaction of some metals with dilute hydrochloric acid, the following observations were made.
(a) Silver metal does not show any change
(b) The temperature of the reaction mixture rises when aluminum (Al) is added.
Answer: a) Because silver is a member of the low reactive series of metals, there will be no reaction between it and dilute hydrogen chloride. b) Due to the fact that it is an exothermic reaction,...
During the reaction of some metals with dilute hydrochloric acid, the following observations were made.
(c) The reaction of sodium metal is found to be highly explosive
d) Some bubbles of gas are seen when lead (Pb) is reacted with the acid.
Answer: c) Sodium is a highly reactive metal, and when it reacts with atmospheric oxygen, it produces an exothermic reaction, which raises the temperature of the surrounding environment. d) Hydrogen...
A substance X, which is an oxide of a group 2 element, is used intensively in the cement industry. This element is present in bones also. On treatment with water, it forms a solution that turns red litmus blue. Identify X and also write the chemical reactions involved.
Answer: Calcium oxide is the chemical compound X. CaO is a compound that is widely used in the cement industry. Water treatment of CaO results in the formation of Ca(OH)2, which is alkaline in...
Write a balanced chemical equation for each of the following reactions and also classify them.
(c) Iron (III) oxide on heating with carbon monoxide gas reacts to form solid iron and liberates carbon dioxide gas.
(d) Hydrogen sulfide gas reacts with oxygen gas to form solid sulfur and liquid water.
Answer: (c) Fe2O3 + 3CO + 2Fe + 3CO2 This is a redox reaction. (d) 2 H2S+O2 → 2 s + 2 H2O This is a replacement reaction.
Write a balanced chemical equation for each of the following reactions and also classify them.
(a) Lead acetate solution is treated with dilute hydrochloric acid to form lead chloride and acetic acid solution.
(b) A piece of sodium metal is added to absolute ethanol to form sodium ethoxide and hydrogen gas.
Answer: (a) Pb(CH3COO)2 + 2HCI – PbCl2 + CH3COOH This is a Double Displacement reaction. (b) 2Na + 2C2H5OH + 2C2H5ONa+ H2 This is a Displacement reaction.
Why do we store silver chloride in dark coloured bottles?
Answer: When exposed to sunlight, silver chloride decomposes into silver and chlorine gas, which are both toxic. As a result, silver chloride is stored in bottles that are dark in colour.
Balance the following chemical equations and identify the type of chemical reaction.
(c) Na(s) + S(s) → Fuse Na2S(s)
(d) TiCl4 (l) + Mg(s) → Ti(s) + MgCl2 (s)
Answer: (c) 2Na(s) + S(s) — (Fuse) → Na2S(s) This is an example of a reaction known as a Combination reaction. (d) TiCI4 (1) + Mg(s) → Ti(s) + 2MgCl2 (s) There are several types of displacement...
Balance the following chemical equations and identify the type of chemical reaction.
(e) CaO(s) + SiO2 (s) → CaSiO3 (s)
(f) H2O2 (l) → U V H2O(l) + O2 (g)
Solution: (e) Cao(s) + SIO2(s) + CaSIO3(s) This reaction falls under the category of Displacement reactions (f) 2H2O2 (I) — UV → 2H2O(I) + O2 (g) This is a decomposition reaction.
Balance the following chemical equations and identify the type of chemical reaction.
(a) Mg(s) + 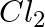 (g) →
(g) → 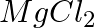 (s)
(s)
(b) HgO(s) → Heat Hg(l) + 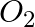 (g)
(g)
Answer: (a) Mg(s) + Cl2(g) → MgCl2(s) A combination reaction, also known as a synthesis reaction, is a type of reaction in which two or more substances are combined. (b) 2HgO(s) — (Heat) → 2 Hg(I) +...
A magnesium ribbon is burnt in oxygen to give a white compound X accompanied by the emission of light. If the burning ribbon is now placed in an atmosphere of nitrogen, it continues to burn and forms a compound Y.
(a) Write the chemical formulae of X and Y.
(b) Write a balanced chemical equation, when X is dissolved in water.
Solution: a) It is formed when magnesium ribbon is burned in oxygen with the emission of light and heat energy that magnesium oxide is formed.X is a compound with the chemical formula MgO. After...
Zinc liberates hydrogen gas when reacted with dilute hydrochloric acid, whereas copper does not. Explain why?
Answer: Copper is more reactive than zinc because zinc is placed above hydrogen in the activity series of metals, whereas copper is placed below hydrogen in the activity series of metals. As a...
A silver article generally turns black when kept in the open for a few days. The article when rubbed with toothpaste again starts shining. (a) Why do silver articles turn black when kept in the open for a few days? Name the phenomenon involved. (b) Name the black substance formed and give its chemical formula.
Solution: A reaction between silver and H2S in the atmosphere results in the formation of Silver Sulphide, which is a dark brown compound with a sulfuric acid smell. Corrosion is the term used to...
On heating blue colored powder of copper (II) nitrate in a boiling tube, copper oxide (black), oxygen gas and a brown gas X are formed
(c) Identify the type of reaction.
(d) What could be the pH range of the aqueous solution of the gas X?
Answer: c) Thermal decomposition is the reaction that is taking place. d) Due to the fact that NO2 dissolves in water and forms an acidic solution, pH is less than 7. (pH range below 7).
