Correct option is (D) Concave, convex Myopia means short-sightedness, in this, a person has a clear vision when looking at objects close to them, but distant objects will appear blurred, to correct...
A …… lens is used to correct myopia and a ….. lens is used to correct hypermetropia.
Rate of reaction of any substance depends on
(A) active mass
(B) molecular weight
(C) atomic weight
(D) equivalent weight
Correct option is (A) active mass The rate at which a substance reacts depends upon its active mass as the rate of reaction is directly proportional to concentration of each reactant and product.
Formula of oleum is
(A) 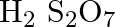
(B) 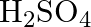
(C) 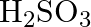
(D) 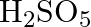
Correct option is (A) $\mathrm{H}_{2} \mathrm{~S}_{2} \mathrm{O}_{7}$ Oleum, or fuming sulfuric acid, is a solution of various compositions of sulfur trioxide in sulfuric acid, or sometimes more...
Two cells of emf 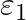 and
and 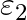 , internal resistance
, internal resistance 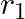 and
and 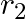 , connected in parallel. The equivalent emf of the combination is- (A)
, connected in parallel. The equivalent emf of the combination is- (A) 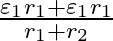 (B)
(B) 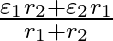 (C)
(C) 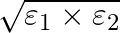 (D)
(D) 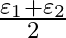
Answer: Option (B)$\frac{\varepsilon_{1} r_{2}+\varepsilon_{2} r_{1}}{r_{1}+r_{2}}$
Acetic acid on treatment with NaOH gives Y and with SOCl2 gives Z. Y and Z when heated form acetic anhydride. The compounds Y and Z respectively are:
Solution: Option 3 is correct Sodium hydroxide (NaOH) reacts with acetic acid (CH3COOH) to form sodium acetate (CH3COONa) and water (H2O). The equation for this reaction can...
Calculate the volume of bcc type unit cell having edge length 288 pm and 7.2 g cm-3 density? (mass of unit cell= 52g)
$ 1.\,\,7.22\,c{{m}^{3}} $ $ 2.\,\,1.38\times {{10}^{-1}}\,c{{m}^{3}} $ $ 3.\,\,2.38\times {{10}^{-2}}\,c{{m}^{3}} $ $ 4.\,\,3.023\times {{10}^{-2}}\,c{{m}^{3}} $ Solution: $ 1.\,\,7.22\,c{{m}^{3}}...
What type of glycosidic linkage is present in maltose ?
1. 1 → 6 β 2. 1 → 4 β 3. 1 → 6 α 4. 1 → 4 α Solution: 1 → 4 α Maltose is a disaccharide made up of alpha 1,4 glucose molecules linked together. Maltase is the enzyme that breaks it down. It acts on...
Which of the following pairs of solutions will be isotonic at the same temperature?
1M NaCl and 1M MgCl2 1.5M KCl and 2.5M Urea 1.5M AlCl3 and 2M Na2SO4 1M NaCl and 2M MgCl2 Solution: 1.5M AlCl3 and 2M Na2SO4 The osmotic pressure and molar concentration in isotonic solutions should...
Identify optically inactive compound among the following
1. 2 - chloropropanal 2. 2 - chloropentane 3. 2 - chloro,2-methylbutane 4. 2 - chlorobutane Solution: 2 - chloro,2-methylbutane 2-chloro-2-methylbutane is optically inactive as no chiral center is...
Which among the following complex ion is NOT diamagnetic?
$ 1.\,\,{{\left[ Ni{{(CN)}_{4}} \right]}^{2-}} $ $ 2.\,\,[Ni{{(CO)}_{4}}] $ $ 3.\,\,{{\left[ CO{{(N{{H}_{3}})}_{6}} \right]}^{3+}} $ $ 4.\,\,{{[NiC{{l}_{4}}]}^{2-}} $ Solution: $...
The standard enthalaphy of formation of ethane is -84.7 KJ/mol. What is the enthalapy change for the formation of 0.06 kg ethane?
1. -42.35 kJ 2. -169.4 kJ 3. -50.82 kJ 4. -236.7 kJ Solution: -169.4 kJ Given mass = 0.06 kg = 60 g Molar mass of ethane = 2(12) + 6(1) = 30 g number of moles of ethane in 60 grams = 60/30 = 2 We...
What happens during the action of catalyst?
1. Temperature increases 2. Temperature decreases 3. Ea increases 4. Ea decreases Solution: Ea decreases A catalyst is a material that speeds up a reaction without being consumed in the process....
The bisectors of angles A and B of a scalene triangle ABC meet at O. (i) What is the point O called? (ii) OR and OQ is drawn a perpendicular to AB and CA respectively. What is the relation between OR and OQ? (iii) What is the relation between ∠ACO and ∠BCO?
Solution: (i) The point O where the angle bisectors meet is called the incenter of the triangle. (ii) The perpendicular drawn from point O to AB and CA are equal. i.e., OR and OQ. (iii) ∠ACO = ∠BCO....
Styrene and 1,3-butadiene on polymerisation forms
1. Buna-N 2. Neoprene 3. Butyl rubber 4. Buna-S Solution: Buna-S Styrene-butadiene rubber is another name for the rubber produced (SBR). Bu stands for butadiene, Na for sodium, and S for styrene in...
Using ruler and compasses only, draw an equilateral triangle of side 5 cm and draw its inscribed circle. Measure the radius of the circle.
Solution: Steps to construct: Step 1: Draw a line segment BC = 5cm. Step 2: With Center as B and radius 5cm, with center as C and radius 5cm draw two arcs which intersect each other at point A. Step...
Construct a tangent to a circle of radius 4cm from a point on the concentric circle of radius 6 cm and measure its length. Also, verify the measurement by actual calculation.
Solution: Steps to construct: Step 1: Mark a point O. Step 2: With center O and radius 4cm and 6cm, draw two concentric circles. Step 3: Join OA and mark its mid-point as M. Step 4: With center M...
The temperature and pressure of 4 dm3 of an ideal gas are doubled. The volume of the gas now is
2 dm3 3 dm3 4 dm3 8 dm3 Solution: 4 dm3 From ideal gas equation, we have: $ \frac{{{P}_{1}}{{V}_{1}}}{{{T}_{1}}}=\frac{{{P}_{2}}{{V}_{2}}}{{{T}_{2}}} $ $ We\,have\,: $ $...
PCl5 exists but NCl5 doesn’t, because of
1. NCl5 is unstable 2. larger size of nitrogen 3. inertness of nitrogen 4. non-availability of vacant d- atomic orbitals Solution: non-availability of vacant d- atomic orbitals By employing the...
Which among following raections of water generates oxygen gas ?
1. Reaction with calcium oxide 2. Reaction with sodium 3. Reaction with ammonia 4. Photosynthesis reaction Solution: Photosynthesis reaction The overall balanced equation of photosynthesis reaction...
a) In the figure (i) given below, O is the centre of the circle. Prove that ∠AOC = 2 (∠ACB + ∠BAC). (b) In the figure (ii) given below, O is the centre of the circle. Prove that x + y = z
Solution : (a) Given: O is the center of the circle. To Prove : ∠AOC = 2 (∠ACB + ∠BAC). Proof: In ∆ABC, ∠ACB + ∠BAC + ∠ABC = 180° (Angles of a triangle) ∠ABC = 180o – (∠ACB + ∠BAC)….(i) In the...
Nitronic acid with 50% sulphuric acid at room temperature forms an aldehyde. This reaction is known as
1. Gabriel phthalamide synthesis 2. Nef carbonyl synthesis 3. Etard reaction 4. Hoffmann degradation Solution: Nef carbonyl synthesis Nef carbonyl synthesis Acid hydrolysis of a salt of a...
(a) In the figure (i) given below, triangle ABC is equilateral. Find ∠BDC and ∠BEC. (b) In the figure (ii) given below, AB is a diameter of a circle with center O. OD is perpendicular to AB and C is a point on the arc DB. Find ∠BAD and ∠ACD
Solution: (a) triangle ABC is an equilateral triangle Each angle = 60o ∠A = 60o But ∠A = ∠D (Angles in the same segment) ∠D = 600 Now ABEC is a cyclic quadrilateral, ∠A = ∠E = 180o 60o + ∠E = 180o...
Three circles of radii 2 cm, 3 cm and 4 cm touch each other externally. Find the perimeter of the triangle obtained on joining the centers of these circles.
Solution: Three circles with centers A, B and C touch each other externally at P, Q and R respectively and the radii of these circles are 2 cm, 3 cm and 4 cm. By joining the centers of triangle ABC...
(a) If a, b, c are the sides of a right triangle where c is the hypotenuse, prove that the radius r of the circle which touches the sides of the triangle is given by r = /frac (a + b – c) – (2) (b) In the given figure, PB is a tangent to a circle with center O at B. AB is a chord of length 24 cm at a distance of 5 cm from the center. If the length of the tangent is 20 cm, find the length of OP.
Solution: (a) Let the circle touch the sides BC, CA and AB of the right triangle ABC at points D, E and F respectively, where BC = a, CA = b and AB = c (as showing in the given figure). As the...
(a) In the figure (i) given below, O is the center of the circle and AB is a tangent at B. If AB = 15 cm and AC = 7.5 cm, find the radius of the circle. (b) In the figure (ii) given below, from an external point P, tangents PA and PB are drawn to a circle. CE is a tangent to the circle at D. If AP = 15 cm, find the perimeter of the triangle PEC.
Solution: (i) Join OB ∠OBA = 90° (Radius through the point of contact is perpendicular to the tangent) OB2 = OA2 – AB2 r2 = (r + 7.5)2 – 152 r2 = r2 + 56.25 + 15r – 225 15r = 168.75 r = 11.25 Hence,...
(a) In figure (i) given below, quadrilateral ABCD is circumscribed; find the perimeter of quadrilateral ABCD. (b) In figure (ii) given below, quadrilateral ABCD is circumscribed and AD ⊥ DC ; find x if radius of incircle is 10 cm.
Solution: (a) From A, AP and AS are the tangents to the circle ∴AS = AP = 6 From B, BP and BQ are the tangents ∴BQ = BP = 5 From C, CQ and CR are the tangents CR = CQ From D, DS and DR are the...
The sodium fusion extract of aniline is boiled with ferrous sulphate and then acidified with concentrated sulphuric acid. The color of the complex formed is
1. Violet 2. Yellow 3. Black 4. Prussian blue Solution: Prussian blue The reaction equation is: $ FeS{{O}_{4}}+NaOH\,\,\to \,\,Fe{{(OH)}_{2}}+N{{a}_{2}}S{{O}_{4}} $ $ 6NaCN+Fe{{(OH)}_{2}}\,\,\to...
Two circles of radii 5 cm and 2-8 cm touch each other. Find the distance between their centers if they touch : (i) externally (ii) internally.
Solution: Radii of the circles are 5 cm and 2.8 cm. i.e. OP = 5 cm and CP = 2.8 cm. (i) When the circles touch externally, then the distance between their centers = OC = 5 + 2.8 = 7.8 cm. (ii) When...
A point P is at a distance 13 cm from the center C of a circle and PT is a tangent to the given circle. If PT = 12 cm, find the radius of the circle.
Solution: CT is the radius CP = 13 cm and tangent PT = 12 cm CT is the radius and TP is the tangent CT is perpendicular TP Now in right angled triangle CPT, CP2 = CT2 + PT2 [using Pythagoras axiom]...
Which among the following elements exhibits only +3 oxidation state?
Nd La Dy Yb Solution: La We have Z = 57 for La. In +3 oxidation state Lanthanum(La) achieves the noble gas configuration. So, Lanthanum exhibits only +3 oxidation state.
(a) In the figure (i) given below, AB is a diameter of the circle. If ∠ADC = 120°, find ∠CAB. (b) In the figure (ii) given below, sides AB and DC of a cyclic quadrilateral ABCD are produced to meet at E, the sides AD and BC are produced to meet at F. If x : y : z = 3 : 4 : 5, find the values of x, y and z.
Solution: (a) Construction: Join BC, and AC then ABCD is a cyclic quadrilateral. Now in ∆DCF Ext. ∠2 = x + z and in ∆CBE Ext. ∠1 = x + y Adding (i) and (ii) x + y + x + z = ∠1 + ∠2 2 x + y + z =...
(a) In the figure given below, PQ is a diameter. Chord SR is parallel to PQ.Given ∠PQR = 58°, calculate (i) ∠RPQ (ii) ∠STP (T is a point on the minor arc SP)
(b) In the figure given below, if ∠ACE = 43° and ∠CAF = 62°, find the values of a, b and c (2007) Solution: (a) In ∆PQR, ∠PRQ = 90° (Angle in a semi-circle) and ∠PQR = 58° ∠RPQ = 90° – ∠PQR = 90° –...
Any galvanic cell working under standard condition, if the equation of the cell reaction is multiplied by 3 then
A. Eo increase three times B. Eo is unchanged C. Eo decreases three times D. Go remains unchanged Solution: Eo is unchanged $ Nernst's\text{ }equation\text{ }states~: $ $...
What type of hybridization is present in PCl5 molecule?
A. sp3d2 B. sp3d3 C. sp3d D. dsp2 Solution: sp3d2 PCl5 is expected to have a hybridization of sp3d and a shape of trigonal bipyramidal. But in solid-state PCl5 exists in ionic form...
(a) In the figure (i) given below, ABCD is a parallelogram. A circle passes through A and D and cuts AB at E and DC at F. Given that ∠BEF = 80°, find ∠ABC. (b) In the figure (ii) given below, ABCD is a cyclic trapezium in which AD is parallel to BC and ∠B = 70°, find: (i)∠BAD (ii) DBCD.
Solution: (a) ADFE is a cyclic quadrilateral Ext. ∠FEB = ∠ADF ⇒ ∠ADF = 80° ABCD is a parallelogram ∠B = ∠D = ∠ADF = 80° or ∠ABC = 80° (b)In trapezium ABCD, AD || BC (i) ∠B + ∠A = 180° ⇒ 70° + ∠A =...
Identify the amphoteric oxide from the following
B2O3 CO ZnO CaO Solution: ZnO Zinc oxide (ZnO) is known as amphoteric oxide because it has both acidic and basic properties.
(a) In the figure (i) given below, M, A, B, N are points on a circle having centre O. AN and MB cut at Y. If ∠NYB = 50° and ∠YNB = 20°, find ∠MAN and the reflex angle MON. (b) In the figure (ii) given below, O is the centre of the circle. If ∠AOB = 140° and ∠OAC = 50°, find (i) ∠ACB (ii) ∠OBC (iii) ∠OAB (iv) ∠CBA
Solution (a) ∠NYB = 50°, ∠YNB = 20°. In ∆YNB, ∠NYB + ∠YNB + ∠YBN = 180o 50o + 20o + ∠YBN = 180o ∠YBN + 70o = 180o ∠YBN = 180o – 70o = 110o But ∠MAN = ∠YBN (Angles in the same segment) ∠MAN = 110o...
(a) In the figure (i) given below, AD || BC. If ∠ACB = 35°. Find the measurement of ∠DBC. (b) In the figure (ii) given below, it is given that O is the centre of the circle and ∠AOC = 130°. Find ∠ ABC
Solution: (a) Construction: Join AB ∠A = ∠C = 350 (Alt Angles) ∠ABC = 35o (b) ∠AOC + reflex ∠AOC = 360o 130o + Reflex ∠AOC = 360o Reflex ∠AOC = 360o – 130o = 230o Now arc BC Subtends reflex ∠AOC at...
Using the given information, find the value of x in each of the following figures:
Solution: (i) ∠ADB and ∠ACB are in the same segment. ∠ADB = ∠ACB = 50° Now in ∆ADB, ∠DAB + X + ∠ADB = 180° = 42o + x + 50o = 180o = 92o + x = 180o x = 180o – 92o x = 88o (ii) In the given figure we...
Which of the following allotropic forms of sulphur exists in chair form?
1. Cyclo - sulphur 2. α - Sulphur 3. β - Sulphur 4. plastic sulphur Solution: Cyclo - sulphur
The standard electode potential of calomel electrode is increased by
decreasing the concentration of KCl solution Increasing the quality of Hg2Cl2 decreasing the quantity of calomel Increasing the concentration of KCl solution Solution: decreasing the concentration...
What is the charge on 0.05 mol of electrons?
1. 2412.5 C 2. 965 C 3. 4825 C 4. 9650 C Solution: 4825 C We have: Mass of one electron = 9.10×10−31 kg Charge of one electron = 1.602×10−19 coulomb $...
Which is most stable oxidation state of Vanadium (Atomic no. 23)?
1. +2 2. +3 3. +4 4. +5 Solution: +5 Ammonium metavanadate, NH4VO3, is the most common source of vanadium in the +5 oxidation state. This isn't particularly water soluble, thus it's normally...
When aniline forms 2,4,6- tribromo aniline on reaction with bromine water , it undergoes
1. nucleophilic addition 2. nucleophilic substitution 3. electrophilic substitution 4. electrophilic addition Solution: electrophilic substitution Bromination is the reaction that is taking place...
For the elementary reaction  . Identify the correct relation from the following relations:
. Identify the correct relation from the following relations:
Solution: the correct option is A As the reaction progresses, the reactant concentration decreases and the product concentration rises. As a result, the rate of...
The decreasing order of thermal stability of hydrides of group 15 elements is
Solution: The correct option is 2. Hydride stability reduces as one moves along the group from NH3 to SbH3. This is because their bond dissociation enthalpy has decreased. The...
The magnitude of 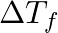 is
is
1. Nature of solute only 2. Nature of the solute and the concentration of solution 3. Nature of the solvent and the concentration of solution 4. Nature of solvent only Solution: 3. Nature of the...
What is SI unit of Luminous intensity?
1. Ampere 2. 0C 3. Kelvin 4. Candela Solution: Candela The SI unit of luminous intensity is candela.
Two moles of an ideal gas are expanded from volume of 15.5 litre to 20 litre against a constant external pressure of 1 atmosphere. The amount of work done is
- 4.5 J 2×10-2 J - 506.5 J 225 J Solution: - 506.5 J The following equation can be used to describe the work done by a system against an external pressure: We know that: w = -Pext ∆V 1atm...
Which of following is a mineral of aluminium?
1. Dolomite 2. Siderite 3. China clay 4. Calamine Solution: China Clay $ Dolomite-[CaMg{{(C{{O}_{3}})}_{2}}] $ $ Siderite-[FeC{{O}_{3}}] $ $ China\text{ }Clay-[Al2{{O}_{3}}.2Si{{O}_{2}}.2{{H}_{2}}O]...
Neo-pentyl alcohol is a –
1. Tertiary alcohol 2. Secondary alcohol 3. Primary alcohol 4. Dihydric alcohol Solution: Primary alcohol The primary alcohols are those that have only one alkyl group linked to the carbon atom of...
Which among following element has highest chemical reactivity?
1. Be 2. Mg 3. Sr 4. Ba Solution: Ba Because of its high reactivity, barium (Ba) is only found in nature in conjunction with other elements. Sulfate and carbonate are the most common compounds...
Which of the following rate expression is true for alkaline hydrolysis of methyl bromide ?
Solution: Option 1. is the correct answer Consider methyl bromide's alkaline hydrolysis to yield methanol. $ C{{H}_{3}}-Br+NaOH\,\,\xrightarrow{\Delta }\,\,C{{H}_{3}}-OH+NaBr $...
Which among following gases is readily adsorbed by activated charcoal ?
H2 SO2 N2 O2 Solution: SO2 SO2 is an easily liquefiable gas and easily liquefiable gases are adsorbed to a greater extent than elemental gases like N2, O2, and H2.
Which one of the following is a neutral oxide?
$ 1.\,A{{l}_{2}}{{O}_{3}} $ $ 2.\,{{N}_{2}}O $ $ 3.\,N{{a}_{2}}O $ $ 4.\,S{{O}_{2}} $ Solution: $ 2.\,{{N}_{2}}O $ Oxides that are neither acidic nor basic are known as neutral oxides. In other...
A polymer obtained from the monomers ethylene glycol and dimethyl terephthalate is
1. Nylon-6 2. Terylene 3. Bakelite 4. Nylon-6,10 Solution: terylene Ethylene glycol (1,2 ethanediol) and terephthalic acid are the monomers of terylene (1,4 benzene dicarboxylic acid). Terylene...
Identify diamagnetic ion from following. (Atomic no.of Na=11, Cu=29, Fe=26, Cr=24)
Cu2+ Fe3+ Na+ Cr3+ Solution: Na+ Cu2+ = 1s22s22p63s23p63d9 Fe3+ = 1s22s22p63s23p63d5 Cr3+ = 1s22s22p63s23p63d3 Na+ = 1s22s22p6 All the given ions except Na+ have unpaired electrons in their...
Identify the oxidising agent in following redox reaction
$ C{{l}_{2}}+2B{{r}^{-}}\,\to \,2C{{l}^{-}}+B{{r}_{2}} $ $ 1.\,C{{l}^{-}} $ $ 2.\,B{{r}^{-}} $ $ 3.\,B{{r}_{2}} $ $ 4.\,C{{l}_{2}} $ Solution: $ 4.\,C{{l}_{2}} $ Br is the reducing agent because it...
Among the following, an artificial sweetening agent which does not contain – CO – NH – bonding in molecule is
1. Sucralose 2. Alitame 3. Aspartame 4. Saccharine Solution: Sucralose Alitame, Aspartame and Saccharine all three contain the CO-NH linkage whereas Sucrolose contains the C-O linkage....
Which of the following nitro-alkane doesn’t react with nitrous acid?
2-methyl-2-nitropropane 2-nitropropane Nitroethane 1-nitropropane Solution: 2-methyl-2-nitropropane The structure of 2-methyl-2-nitropropane is as follows: We can see that...
Pumice stone is an example of
Solid Sol Emulsion Aerosol Solid foam Solution: Solid Foam Pumice stone is an example of solid foam. In this type of colloid, the dispersion medium is solid and the dispersion phase is...
 molecules of urea are present in 100 mL of its solution. The concentration of solution is
molecules of urea are present in 100 mL of its solution. The concentration of solution is
0.1 M 0.02 M 0.01 M 0.001 M Solution: 0.01 M Given: 6.02×1020 molecules of urea The volume of solution is 100/1000 = 0.1 L 1 mole of urea have 6.02×1023 molecules Number of moles present = 6.02×1020...
Identify the product B in following conversion!
$Chlorobenzene+{{H}_{2}}O\xrightarrow[\Pr essure]{Cu,\,673K}A\xrightarrow[373K]{conc.\,{{H}_{2}}S{{O}_{4}}}B$ 4-hydroxybenzene Sulphonic acid Benzene Sulphonic acid 2-hydroxybenzene Sulphonic acid...
What is the quantity of Gold chloride obtained when 4.5 g of gold and 2.1 g of Chlorine are sealed in a a tube and heated at 150 degreees C?
4.5 g 4.8 g 6.07 g 20.7 g Solution: 6.07 g Excess reagents are reactants that are not used up when a chemical reaction is completed. Because its quantity limits the amount of product generated, the...
What is the standard emf of following cell?
$ N{{i}_{(s)}}|(1M)\,Ni_{(aq)}^{2+}||(1M)\,Au_{(aq)}^{3+}|Au\left( s \right) $ $ if\,E_{Ni}^{\circ }=-0.25V,\,E_{Au}^{\circ }=1.50V $ -1.25 V 1.75 V 1.25 V -1.75 V Solution: 1.75 V $...
5600 sec 360.0 sec 560.0 sec 3364 sec Solution: 560 sec $ Given: $ $ {{[R]}_{0}}=0.0210\,M~ $ $ [R]=0.0150\,M~ $ $ k=6\times {{10}^{-4}}\,{{\sec }^{-1}} $ $ For\text{ }a\text{ }first\text{...
Which of the following alcohols is not having Csp3-OH bond?
Phenyl Methanol 2-Methyl Propan-2-ol Propan-2-ol Vinyl Alcohol Solution: Vinyl Alcohol Vinyl Alcohol is represented as follows: The simplest enol is vinyl alcohol, commonly...
What is the number of hydroxyl group present in lactic acid?
Zero Three Two One Solution: One Lactic acid (2-hydroxy propionic acid) is a bifunctional molecule that has both a carboxylic acid and a hydroxyl group, making it useful in a...
Which of the following is not an octahedral complex?
$ 1.\,\,{{[Ir{{({{C}_{2}}{{O}_{4}})}_{2}}C{{l}_{2}}]}^{3-}} $ $ 2.\,\,{{[CoC{{l}_{2}}{{(en)}_{2}}]}^{+}} $ $ 3.\,\,{{\left[ Co{{(en)}_{2}}{{(N{{O}_{3}})}_{2}} \right]}^{+}} $ $ 4.\,\,\left[...
What is the oxidation state of iron in potassium ferrate?
+3 +4 +6 +2 Solution: +6 Potassium ferrate has the chemical formula K2FeO4. This purple salt is paramagnetic, and is a rare example of an iron(VI) compound. $...
What is the number of moles of Silver Chloride precipitated when excess of aqueous silver nitrate is treated with ![Rendered by QuickLaTeX.com [Co{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]Cl](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-7f8a1797a451d4bad014fffc46745e19_l3.png)
1 mole 2 mole 3 mole 4 mole Solution: 1 mole Only one mole of AgCI is precipitated with an excess of AgNO3 because only one Cl- is in the ionization sphere.
Which of the following is not found in hybridization?
Formation of sigma bonds Mixing and recasting of atomic orbitals Excitation of electrons Loss and gain of electrons Solution: Loss and gain of electrons The following ideas are important in...
Which of the following statements is true for pyran?
It is a saturated aliphatic compound, It is a homocyclic compound. It is a heterocyclic compound with oxygen atom in the ring The molecular formula of Pyran is C5H5S Solution: It is a heterocyclic...
Which of the following elements has six unpaired electrons in observed electronic observations?
Fe (Z = 26) Cr (Z = 24) Cu (Z = 29) Mn (Z = 25) Solution: Cr (Z = 24) The following is the electrical configuration of the given elements: $...
Which of the following is the strongest reducing agent?
Na Mg Li Ca Solution: Li Lithium has the highest ionization potential of all alkali metals, implying that its tendency to ionize to give Li+ ions should be the least, implying that Li should be the...
Which among the following electrical properties has SI unit siemens per meter?
Conductance Conductivity Resistance Resistivity Solution: Conductivity The ability of an electrolyte solution to conduct electricity is measured by its conductivity (or specific conductance)....
Aniline reacts with bromine water at room temperature to give
3-Bromoaniline 2-Bromoaniline 4-Bromoaniline 2,4,6-Tribromoaniline Solution: 2,4,6-Tribromoaniline This happens due to the highly activating nature of -...
Which of the following statements is not true for glyceraldehyde?
It is a sugar molecule It is optically active It contains two asymmetric carbon atoms It has carbonyl and hydroxyl group Solution: It contains two asymmetric carbon atoms ...
Copper crystallizes as face centered cubic lattice , with edge length of unit cell 361 pm. Calculate the radius of the copper atom.
108.6 pm 127.65 pm 181.6 pm 157.6 pm Solution: 127.65 pm For F.C.C, a√2 = 4r 361×√2 = 4r r = 127.65 pm
What is the molecular formula of allyl bromide?
$ 1.\,\,{{C}_{2}}{{H}_{4}}Br $ $ 2.\,\,{{C}_{2}}{{H}_{3}}Br $ $ 3.\,\,{{C}_{3}}{{H}_{5}}Br $ $ 4.\,\,{{C}_{3}}{{H}_{6}}Br $ Solution: $ 3.\,\,{{C}_{3}}{{H}_{5}}Br $ $ The\text{ }Allyl\text{...
Figure shows three forces 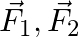 and
and 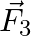 acting along the sides of an equilateral triangle. If the total torque acting at point
acting along the sides of an equilateral triangle. If the total torque acting at point  ‘ (centre of the triangle) is zero then the magnitude of
‘ (centre of the triangle) is zero then the magnitude of 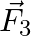 is
is
A)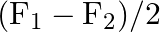
B)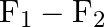
C)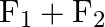
D)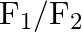
Correct option is C ($\mathrm{F}_{1}+\mathrm{F}_{2}$) Explanation: On taking clockwise $ \begin{array}{l} \mathrm{T}=\mathrm{T}_{\mathrm{F} 1}+\mathrm{T}_{\mathrm{F} 2}+\mathrm{T}_{\mathrm{F} 3} \\...
What is the bond length of C-H bonds in alkanes?
154 pm 120 pm 133pm 112pm Solution: 112 pm
Which of the following solution will have highest freezing point depression?
M Glucose M Sucrose M Urea M KCl Solution: M KCl ΔTf = i Kf m ΔTf is the freezing point depression, i is the van’t Hoff factor, Kf is the molal freezing point depression constant for the solvent,...
Identify the polymer from following, that contains amide linkage
Terylene PHBV Nylon - 6,6 Dextron Solution: Nylon-6,6 Polyamides have the amide linkage. Example- Nylon 6-6, Nylon 6 Nylon 6, 6 is used in the manufacturing of sheets, brush bristles, and...
The volume of 400cm3 of chlorine gas at 400 mm of Hg is decreased to 200 cm3 at constant temperature. What is new pressure of the gas?
800 mm of Hg 1600 mm of Hg 200 mm of Hg 600 mm of Hg Solution: 800 mm of Hg This is an example of Boyle's law, which states that the volume of a gas is inversely proportional to the pressure at...
The H-H bond energy is 430 KJ/mol and Cl-Cl bond energy is 240 KJ/mol. 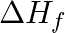 for HCl is 90 KJ/mol. Then HCl bond energy is
for HCl is 90 KJ/mol. Then HCl bond energy is
360 KJ/mol 213 KJ/mol 180 KJ/mol 425 KJ/mol Solution: 425 KJ/mol $ \frac{1}{2}{{H}_{2}}(g)+\frac{1}{2}C{{l}_{2}}(g)\,\,\to \,\,HCl $ $ \Delta {{H}_{f}}=-90KJ $ $ \Delta {{H}_{f}}=~B.E\text{...
Van’t Hoff factor (i) for the centimolal solution of ![Rendered by QuickLaTeX.com {{\text{K}}_{\text{3}}}\text{ }\!\![\!\!\text{ Fe(CN}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-1e79ee193daba828d4bfe9ad8e4b1525_l3.png) is 3.333. What is it’s percentage dissociation?
is 3.333. What is it’s percentage dissociation?
80% 70% 33.33% 77.7% Solution: 77.7% $ {{K}_{3}}[Fe{{(CN)}_{6}}]\to 3{{K}^{+}}+{{[Fe{{(CN)}_{6}}]}^{3-}} $ $ We\,get: $ $ i=\alpha n+(1-\alpha )=1+\alpha (n-1)=1+\alpha (4-1)~ $ $ Van't\text{...
What is the melting point of zinc?
473 K 1193 K 423 K 692 K Solution: 692 K The metal Zinc has a melting point of 692K.
In Merck’s method, hydrogen peroxide is obtained from?
$ 1.\,\,Ba{{O}_{2}}+{{H}_{2}}S{{O}_{4}} $ $ 2.\,\,N{{a}_{2}}{{O}_{2}}+{{H}_{2}}S{{O}_{4}} $ $ 3.\,\,Ba{{O}_{2}}+{{H}_{3}}P{{O}_{4}} $ $ 4.\,\,Ba{{O}_{2}}+{{H}_{2}}O+C{{O}_{2}} $ Solution: $...
An element crystallizes in bcc structure. The number of unit cells of an element in 4 g of it is ( given mass = 40)
0.1 NA / 2 2(0.1) NA 0.1 NA 2 NA Solution: 0.1 NA / 2 Given: Mass of element = 40 g and Z = 2 (bcc structure) The number of moles in 1 gram of element = 1/40 Similarly, number of moles in 4 gram of...
Which of the following is NOT correct in hybridisation?
There should be very little difference in energy of involving orbitals The shape of hybrid orbitals is the same as that of atomic orbitals The number of hybrid orbitals formed is equal to the...
Dumas method is used for the estimation of
Nitrogen Sulphur Oxygen Carbon Solution: Nitrogen In analytical chemistry, the Dumas method is a method for quantifying nitrogen in chemical substances based on a method proposed by Jean-Baptiste...
Which of the following compounds is obtained when 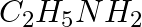 is treated with excess
is treated with excess 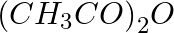 in presence of pyridine?
in presence of pyridine?
$ 1.\,\,{{C}_{2}}{{H}_{5}}N{{(COC{{H}_{3}})}_{2}} $ $ 2.\,\,{{\left( {{C}_{2}}{{H}_{5}} \right)}_{2}}NH $ $ 3.\,\,{{C}_{2}}{{H}_{5}}COOH $ $ 4.\,\,{{C}_{2}}{{H}_{5}}NHCOC{{H}_{3}} $ Solution: $...
Which among the following polymers is an example of addition polymers?
Dacron Unrealformaldehyde Polymer Nylon-6 Polythene Solution: Polythene The addition polymers are formed by the repeated addition of monomer molecules possessing double or triple bonds, e.g., the...
Which among the following is a first oxidation product of butan-2-ol?
Butanal Butanoic Acid Propanoic acid and carbon di-oxide Butan-2-one Solution: Butan-2-one Ketone is the first oxidation product of secondary alcohol. For example, butan-2-ol is oxidised to...
Which of the following compounds is obtained when benzene is treated with CO and HCl in presence of catalyst anhyfrous  under high pressure?
under high pressure?
Toulene Benzoic Acid Benzaldehyde Acetophenone Solution: Benzaldehyde When the vapors of CO and HCI are passed into benzene in the presence of anhydrous AICI3/CuCI, benzaldehyde...
Which among the following oxides of nitrogen, the nitrogen atom contains one unpaired electron?
$ A)\,{{N}_{2}}{{O}_{4}} $ $ B)\,N{{O}_{2}} $ $ C)\,{{N}_{2}}{{O}_{5}} $ $ D)\,{{N}_{2}}{{O}_{3}} $ Solution: $ B)\,N{{O}_{2}} $ Among the oxides of nitrogen, only NO2 contains odd number of...
The rate of first order reaction is A->B is 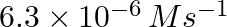 . if [A] = 0.3 M what is the arye
. if [A] = 0.3 M what is the arye
$ A)\,\,2.1\times {{10}^{-5}}\,{{s}^{-1}} $ $ A)\,\,1.2\times {{10}^{-5}}\,{{s}^{-1}} $ $ A)\,\,1.3\times {{10}^{-5}}\,{{s}^{-1}} $ $ A)\,\,1.6\times {{10}^{-5}}\,{{s}^{-1}} $ Solution: $...
In a ∆ABC, D and E are points on the sides AB and AC respectively such that AD = 5.7cm, BD = 9.5cm, AE = 3.3cm and AC = 8.8cm. Is DE || BC? Justify your answer.
Solution:- From the question it is given that, In a ∆ABC, D and E are points on the sides AB and AC respectively. AD = 5.7cm, BD = 9.5cm, AE = 3.3cm and AC = 8.8cm Consider the ∆ABC, EC = AC – AE =...
In the adjoining figure, AB = AC. If PM ⊥ AB and PN ⊥ AC, show that PM x PC = PN x PB.
Solution:- From the given figure, AB = AC. If PM ⊥ AB and PN ⊥ AC We have to show that, PM x PC = PN x PB Consider the ∆ABC, AB = AC … [given] ∠B = ∠C Then, consider ∆CPN and ∆BPM ∠N = ∠M … [both...
Given that ∆s ABC and PQR are similar. Find: (i) The ratio of the area of ∆ABC to the area of ∆PQR if their corresponding sides are in the ratio 1 : 3. (ii) the ratio of their corresponding sides if area of ∆ABC : area of ∆PQR = 25 : 36.
Solution:- From the question it is given that, (i) The area of ∆ABC to the area of ∆PQR if their corresponding sides are in the ratio 1 : 3 Then, ∆ABC ~ ∆PQR area of ∆ABC/area of ∆PQR = BC2/QR2 So,...
In the figure (ii) given below, PQ || AC, AP = 4 cm, PB = 6 cm and BC = 8 cm. Find CQ and BQ.
Solution:- From the figure, PQ || AC, AP = 4 cm, PB = 6 cm and BC = 8 cm ∠BQP = ∠BCA … [because alternate angles are equal] Also, ∠B = ∠B … [common for both the triangles] Therefore, ∆ABC ~ ∆BPQ...
In the given figure, CM and RN are respectively the medians of ∆ABC and ∆PQR. If ∆ABC ~ ∆PQR, prove that:
(i) ∆AMC ~ ∆PQR
(ii) CM/RN = AB/PQ
(iii) ∆CMB ~ ∆RNQ
Solution:- From the given figure it is given that, CM and RN are respectively the medians of ∆ABC and ∆PQR. (i) We have to prove that, ∆AMC ~ ∆PQR Consider the ∆ABC and ∆PQR As ∆ABC ~ ∆PQR ∠A = ∠P,...
In the given figure, DB ⊥ BC, DE ⊥ AB and AC ⊥ BC. Prove that BE/DE = AC/BC
Solution:- From the figure, DB ⊥ BC, DE ⊥ AB and AC ⊥ BC We have to prove that, BE/DE = AC/BC Consider the ∆ABC and ∆DEB, ∠C = 90o ∠A + ∠ABC = 90o [from the figure equation (i)] Now in ∆DEB ∠DBE +...
(a) In the figure (1) given below, AP = 2PB and CP = 2PD.
(i) Prove that ∆ACP is similar to ∆BDP and AC || BD.
(ii) If AC = 4.5 cm, calculate the length of BD.
Solution:- From the question it is give that, AP = 2PB, CP = 2PD (i) We have to prove that, ∆ACP is similar to ∆BDP and AC || BD AP = 2PB AP/PB = 2/1 Then, CP = 2PD CP/PD = 2/1 ∠APC = ∠BPD [from...
A card is drawn from a well-shuffled pack of 52 cards. Find the probability of getting:
(v) a king or a queen
(vi) a non-face card
(v) Let E be the event of getting a king or a queen. There will be 4 cards of king and 4 cards of queen. Number of favourable outcomes = 4+4 = 8 P(E) = 8/52 = 2/13 Hence the probability of getting a...
How many spherical lead shots of diameter 4 cm can be made out of a solid cube of lead whose edge measures 44 cm?
Solution: Edge of the cube, a = 44 cm Volume of cube = a3 = 443 = 85184 cm3 Diameter of shot = 4 cm So radius of shot, r = 4/2 = 2 cm Volume of a shot = (4/3)r3 = (4/3)×(22/7)×23 = 704/21 cm3 Number...
Two spheres of the same metal weigh 1 kg and 7 kg. The radius of the smaller sphere is 3 cm. The two spheres are melted to form a single big sphere. Find the diameter of the big sphere.
Solution: For same material, density will be same. Density = mass/Volume Mass of the smaller sphere, m1 = 1 kg Mass of the bigger sphere, m2 = 7 kg The spheres are melted to form a new sphere. So...
A solid metallic hemisphere of radius 8 cm is melted and recasted into right circular cone of base radius 6 cm. Determine the height of the cone.
Solution: Given radius of the hemisphere, r = 8 cm Volume of the hemisphere, V = (2/3)r3 = (2/3)×83 = (1024/3) cm3 Radius of cone, R = 6 cm Since hemisphere is melted and recasted into a cone, the...
An exhibition tent is in the form of a cylinder surmounted by a cone. The height of the tent above the ground is 85 m and the height of the cylindrical part is 50 m. If the diameter of the base is 168 m, find the quantity of canvas required to make the tent. Allow 20% extra for folds and stitching. Give your answer to the nearest m².
solution; Given height of the tent above the ground = 85 m Height of the cylindrical part, H = 50 m height of the cone, h = 85-50 h = 35 m Diameter of the base, d = 168 m Radius of the base of...
From a cube of edge 14 cm, a cone of maximum size is carved out. Find the volume of the remaining material.
Given edge of the cube, a = 14 cm Radius of the cone, r = 14/2 = 7 cm Height of the cone, h = 14 cm Volume of the cube = a3 = 143 = 14×14×14 = 2744 cm3 Volume of the cone = (1/3)r2h =...
Find the surface area of a sphere of radius : (i) 14 cm (ii) 10.5 cm
Solution: (i) Given radius of the sphere, r = 14 cm Surface area of the sphere = 4r2 = 4×(22/7)×142 = 4×22×14×2 = 2464 cm3 Hence the surface area of the sphere is 2464 cm2. (ii) Given radius of the...
The volume of a right circular cone is 9856 cm3 and the area of its base is 616 cm2 . Find
(i) the slant height of the cone.
(ii) total surface area of the cone.
Solution: Given base area of the cone = 616 cm2 r2 = 616 (22/7)×r2 = 616 r2 = 616×7/22 r2 = 196 r = 14 Given volume of the cone = 9856 cm3 (1/3)r2h = 9856 (1/3)×(22/7)×142 ×h = 9856 h =...
A conical tent is 10 m high and the radius of its base is 24 m. Find : (i) slant height of the tent. (ii) cost of the canvas required to make the tent, if the cost of 1 m2 canvas is Rs 70.
Solution: (i)Given height of the tent, h = 10 m Radius, r = 24 m We know that l2 = h2+r2 l2 = 102+242 l2 = 100+576 l2 = 676 l = √676 l = 26 (ii) Curved surface area = rl = (22/7)×24×26 = 13728/7 m2...
If the volume of a right circular cone of height 9 cm is 48π cm3 , find the diameter of its base.
Solution: Given height of a cone, h = 9 cm Volume of the cone = 48 (1/3)r2h = 48 (1/3)r2×9 = 48 3r2 = 48 r2 = 48/3 = 16 r = 4 So diameter = 2×radius = 2×4 = 8 cm Hence the diameter of the cone is 8...
Find the curved surface area of a right circular cone whose slant height is 10 cm and base radius is 7 cm.
Solution: Given slant height of the cone, l = 10 cm Base radius, r = 7 cm Curved surface area of the cone = rl = (22/7)×7×10 = 220 cm2 Hence the curved surface area of the cone is 220...
Find the equation of the line which is parallel to 3x – 2y = – 4 and passes through the point (0, 3).
Solution: Given line: 3x – 2y = -4 Slope (m1) is given by 2y = 3x + 4 y = (3/2) x + 2 So, m1 = 3/2 Now, the slope of the line parallel to the given line will have the same slope as 3/2 = m And the...
The co-ordinates of two points A and B are ( – 3, 3) and (12, – 7) respectively. P is a point on the line segment AB such that AP : PB = 2 : 3. Find the co-ordinates of P.
Solution: Let the co-ordinates of P(x, y) divides AB in the ratio m:n. A(-3,3) and B(12,-7) are the given points. Given m:n = 2:3 x1 = -3 , y1 = 3 , x2 = 12 , y2 = -7 , m = 2 and n = 3 By Section...
Lifetimes of the molecules in the excited states are often measured by using pulsed radiation source of duration nearly in the nanosecond range. If the radiation source has the duration of 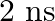 and the number of photons emitted during the pulse source is
and the number of photons emitted during the pulse source is 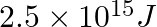 , calculate the energy of the source.
, calculate the energy of the source.
Frequency of radiation $(\nu)$, $\nu=\frac{1}{2.0 \times 10^{-9} s}$ $\nu=5.0 \times 10^{8} s^{-1}$ Energy $(E)$ of source $=$ Nhv Where, $N$ is the no. photons emitted $\mathrm{h}$ is Planck's...
Following results are observed when sodium metal is irradiated with different wavelengths. Calculate threshold wavelength and Planck’s constant.
If the threshold wavelength is $\lambda_{0} n m\left(=\lambda_{0} \times 10^{-9} \mathrm{~m}\right)$, the K.E. of the radiation would be: $ h\left(\nu-\nu_{0}\right)=\frac{1}{2} m \nu^{2} $ Three...
The work function for the caesium atom is 1.9 eV. Calculate
(a) the threshold wavelength and
(b) the threshold frequency of the radiation. If the caesium element is irradiated with a wavelength of 500 nm,(c) calculate the kinetic energy and the velocity of the ejected photoelectron.
Given, the work function of caesium $\left(W_{0}\right)=1.9 \mathrm{eV}$ (a)From the $W_{0}=\frac{h c}{\lambda_{0}}$ expression, we get: $\lambda_{0}=\frac{h c}{W_{0}}$ Where, $\lambda_{0}$ is the...
In astronomical observations, signals observed from the distant stars are generally weak. If the photon detector receives a total of  from the radiations of
from the radiations of 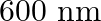 , calculate the number of photons received by the detector.
, calculate the number of photons received by the detector.
From the expression of energy of one photon (E), $ E=\frac{h c}{\lambda} $ Where, $\lambda$ denotes the wavelength of the radiation $\mathrm{h}$ is Planck's constant c denotes the velocity of the...
Neon gas is generally used in the signboards. If it emits strongly at 616 nm, calculate the number of quanta presents if it produces 2 J of energy.
No. quanta in $2 \mathrm{~J}$ of energy $\frac{2 J}{32.27 \times 10^{-20} J}$ $ =6.19 \times 10^{18} $ $ =6.2 \times 10^{18} $
Neon gas is generally used in the signboards. If it emits strongly at 616 nm, calculate the energy of quantum
Energy of one quantum $(E)=h v$ $ =\left(6.626 \times 10^{-34} \mathrm{Js}\right)\left(4.87 \times 10^{14} \mathrm{~s}^{-1}\right) $ Energy of one quantum $(\mathrm{E})=32.27 \times 10^{-20}...
Neon gas is generally used in the signboards. If it emits strongly at 616 nm, calculate distance travelled by this radiation in 30 s
Speed of the radiation, $\mathrm{c}=3 \times 10^{8} \mathrm{~ms}^{-1}$ Distance travelled by the radiation in a timespan of $30 \mathrm{~s}$ $ \begin{array}{l} =\left(3 \times 10^{8}...
Neon gas is generally used in the signboards. If it emits strongly at 616 nm, calculate the frequency of emission,
Wavelength of the emitted radiation $=616 \mathrm{~nm}=616 \times 10^{-9} \mathrm{~m}$ (Given) (a)Frequency of the emission $(\nu)$ $ \nu=\frac{c}{\lambda} $ Where, $c=$ speed of the radiation...
Nitrogen laser produces radiation at a wavelength of 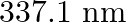 . If the number of photons emitted is
. If the number of photons emitted is 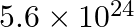 , calculate the power of this laser.
, calculate the power of this laser.
Energy with which it emits photons=Power of laser Power $=E=\frac{N h c}{\lambda}$ Where, $N=$ number of photons emitted $\mathrm{h}=$ Planck's constant $\mathrm{c}=$ velocity of radiation...
Arrange the following type of radiations in increasing order of frequency: (a) radiation from microwave oven (b) amber light from traffic signal (c) radiation from FM radio (d) cosmic rays from outer space and (e) X-rays.
The following is the frequency order in ascending order: Radiation from FM radio < amber light < radiation from microwave oven < X- rays < cosmic rays The following is the increasing...
An ion with mass number 56 contains 3 units of positive charge and 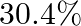 more neutrons than electrons. Assign the symbol to this ion.
more neutrons than electrons. Assign the symbol to this ion.
Let us consider the total no. electrons present in $A^{3+}$ be $x$. Now, total no. neutrons in it $=x+30.4 \%$ of $x=1.304 x$ Since the ion has a charge of $+3, \Rightarrow$ no. electrons in neutral...
An ion with mass number 37 possesses one unit of negative charge. If the ion contains 11.1% more neutrons than the electrons, find the symbol of the ion.
The number of electrons in a negatively charged ion be $x$. Then, no. neutrons present $=x+11.1 \%$ of $x=x+0.111 x=1.111 x$ No. electrons present in the neutral atom $=(x-1)$ (When an ion carries...
An element with mass number 81 contains 31.7% more neutrons as compared to protons. Assign the atomic symbol.
Let us consider that the No.of protons in the element be $x$. No. of neutrons $=x+31.7 \%$ of $x$ $=x+0.317 x$ $=1.317 x$ According to the question, Mass number of the element $=81$, which...
Symbols 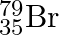 and
and 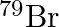 can be written, whereas symbols
can be written, whereas symbols 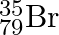 and
and 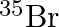 are not acceptable. Answer briefly.
are not acceptable. Answer briefly.
The general convention followed while representing elements along with their atomic masses (A), and their atomic numbers $(Z)$ is ${ }_{Z}^{A} \mathrm{X}$. Therefore, ${ }_{35}^{79} \mathrm{Br}$ is...
In Rutherford’s experiment, generally the thin foil of heavy atoms, like gold, platinum etc. have been used to be bombarded by the α-particles. If the thin foil of light atoms like Aluminium etc. is used, what difference would be observed from the above results?
The findings obtained with a foil made up of heavy atoms will differ from those obtained with a foil made up of comparatively light atoms. The magnitude of positive charge in the nucleus of a...
In Milikan’s experiment, the static electric charge on the oil drops has been obtained by shining 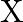 rays. If the static electric charge on the oil drop is
rays. If the static electric charge on the oil drop is  , calculate the number of electrons present on it.
, calculate the number of electrons present on it.
Charge held by the oil drop $=1.282 \times 10^{-18} C$ Charge held by one electron $=1.6022 \times 10^{-19} C$ Therefore, No. electrons present in the drop of oil $\frac{1.282 \times 10^{-18}...
A certain particle carries 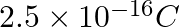 of static electric charge. Calculate the number of electrons present in it.
of static electric charge. Calculate the number of electrons present in it.
Charge held by one electron $=1.6022 \times 10^{-19} C \Rightarrow 1.6022 \times 10^{-19} C$ charge is held by one electron. Therefore, electrons carrying charge of $2.5 \times 10^{-16} C \quad...
The diameter of the zinc atom is 2.6Å. Calculate (a) radius of zinc atom in pm and (b) number of atoms present in a length of 1.6 cm if the zinc atoms are arranged side by side lengthwise.
Radius of carbon atom$ =\frac{2.6}{2} $ $ \begin{array}{l} =1.3 \times 10^{-10} \mathrm{~m} \\ =130 \times 10^{-12} \mathrm{~m}=130 \mathrm{pm} \end{array} $ (b) Length of the arrangement $=1.6...
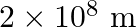 atoms of carbon are arranged side by side. Calculate the radius of carbon atom if th length of this arrangement is
atoms of carbon are arranged side by side. Calculate the radius of carbon atom if th length of this arrangement is 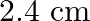 .
.
Length of the arrangement $=2.4 \mathrm{~cm}$ No. carbon atoms present $=2 \times 10^{8}$ The diameter of the carbon atom $=\frac{2.4 \times 10^{-2} m}{2 \times 10^{8} m}$ $=1.2 \times 10^{-10} m...
If the diameter of a carbon atom is 0.15 nm, calculate the number of carbon atoms which can be placed side by side in a straight line across the length of the scale of length 20 cm long.
We know that$ 1 \mathrm{~cm}=10^{-2} \mathrm{~m} $ Length of the scale $=20 \mathrm{~cm}=20 \times 10^{-2} \mathrm{~m}$ Diameter of one carbon atom $=0.15 \mathrm{~nm}=0.15 \times 10^{-9}...
Calculate the energy required for the process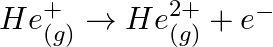 The ionization energy for the
The ionization energy for the 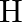 atom in the ground state is
atom in the ground state is 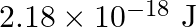 atom
atom 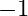
The energy associated with hydrogen-like species is: $ E_{n}=-2.18 \times 10^{-18}\left(\frac{Z^{2}}{n^{2}}\right) J $ For the ground state of the hydrogen atom, $ \begin{array}{l} \Delta...
What transition in the hydrogen spectrum would have the same wavelength as the Balmer transition n = 4 to n = 2 of He+ spectrum?
The wave number associated with the Balmer transition for the He+ ion (n = 4 to n = 2 ) is given by: $ \bar{\nu}=\frac{1}{\lambda}=R Z^{2}\left(\frac{1}{n_{1}^{2}}-\frac{1}{n_{2}^{2}}\right) $...
Show that the circumference of the Bohr orbit for the hydrogen atom is an integral multiple of the de Broglie wavelength associated with the electron revolving around the orbit.
Only one electron exists in hydrogen atoms. The angular momentum of this electron, according to Bohr's postulates, is: $m v r=n \frac{h}{2 \pi} \quad \ldots .(1)$ Where, $n=1,2,3, \ldots$ As per de...
How many electrons in an atom may have the following quantum numbers?a) n = 4, 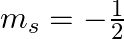 b)n = 3, l = 0
b)n = 3, l = 0
(a)The total number of electrons in the atom =$2n^2$ if n is the primary quantum number. Hence, For n = 4, Total no. electrons = 2 (16) = 32 An atom with 32 electrons has the following electron...
Explain, giving reasons, which of the following sets of quantum numbers are not possible.
a) 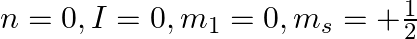
b) 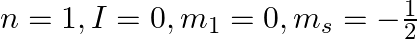
c) 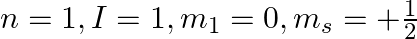
d) 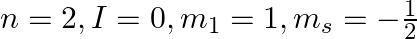
e) 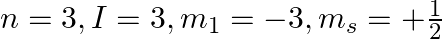
f) 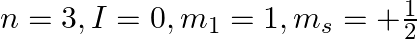
a) This is not possible. The number n cannot be zero. (b) Possible. (c) This is not possible. The value of l can't be the same as the value of n. (d) This is not possible. Because mt can't be 1 when...
Using s, p and d notations, describe the orbital with the following quantum numbers.
(a)n = 1, l = 0;
(b)n = 3; l =1
(c) n = 4; l = 2;
(d) n = 4; l =3.
(a)n = 1, l = 0 implies a 1s orbital. (b)n = 3 and l = 1 implies a 3p orbital. (c)n = 4 and l = 2 implies a 4d orbital. (d)n = 4 and l = 3 implies a 4f orbital.
(I)An atomic orbital has n = 3. What are the possible values of l and ml ? (II)List the quantum numbers (ml and l) of electrons for 3d orbital. (III) Which of the following orbitals are possible? 1p, 2s, 2p and 3f
(I) The range of potential values for 'l' is 0 to (n – 1). As a result, the possible values of l for n = 3 are 0, 1, and 2. The total number of potential ml = (2l + 1) values. It has a range of...
Give the number of electrons in the species , 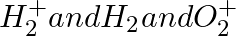
The number of electrons in H2 is 1 + 1 = 2. 2 – 1 = 1 number of electrons in H2+ H2: The number of electrons in H2 equals 1 + 1 = 2. Number of electrons in O2 = 8 + 8 = 16. In O2+, the number of...
An atom of an element contains 29 electrons and 35 neutrons. Deduce (i) the number of protons and (ii) the electronic configuration of the element.
(i)No.protons = no.electrons in a neutral atom. The number of protons in the atoms of the element is 29. (ii)The electronic configuration of this element (atomic number 29) is 1s...
An electron is in one of the 3d orbitals. Give the possible values of n, l and ml for this electron.
For the 3d orbital: Principal quantum number ,possible values (n) = 3 Azimuthal quantum number, possible values(l) = 2 Magnetic quantum number ,possible values(ml) = – 2, – 1, 0, 1, 2
What is the lowest value of n that allows g orbitals to exist?
For g-orbitals, l = 4. The possible values of ‘l’ range from 0 to (n-1),. For any given value of ‘n’, Hence, least value of n = 5, l = 4 (g orbital),
(III) Which atoms are indicated by the following configurations? (a) ![Rendered by QuickLaTeX.com [\mathrm{He}]{2} \mathrm{~s}^{1}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-949e215e254ffdae25444046a6badfbf_l3.png)
 Ne]
Ne] 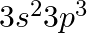 (c)
(c) ![Rendered by QuickLaTeX.com [\mathrm{Ar}] 4 \mathrm{~s}^{2} 3 \mathrm{~d}^{1}](https://www.learnatnoon.com/s/wp-content/ql-cache/quicklatex.com-c46b18eb49590f81f622e0747e88d18b_l3.png)
$(I I I)(a)[H e] 2 s^{1}$ Complete electronic configuration: $1 \mathrm{~s}^{2} 2 \mathrm{~s}{ }^{1}$. $\therefore$ the element's atomic number is 3 . The element is lithium (Li) (b) $[\mathrm{Ne}]...
(II) What are the atomic numbers of elements whose outermost electrons are represented by (a)  (b)
(b) 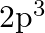 and (c)
and (c) 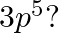
(II) (a) $3 \mathrm{~s}^{1}$ Complete electronic configuration: $1 \mathrm{~s}^{2} 2 \mathrm{~s}^{2} 2 \mathrm{p}^{6} 3 \mathrm{~s}^{1}$ Total no. electrons in the atom $=2+2+6+1=11 \quad...
(I)Write the electronic configurations of the following ions:(a) 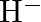 (b)
(b) 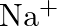 (c)
(c) 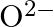 (d)
(d) 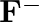
The electronic configuration of the Hydrogen atom (in its ground state) $1 \mathrm{~s}^{1}$. The single negative charge on this atom indicates that it has gained an electron. Hence, the electronic...
The mass of an electron is 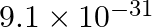 kg. If its K.E. is
kg. If its K.E. is J, calculate its wavelength.
J, calculate its wavelength.
As per de Broglie’s equation, $ \lambda=\frac{h}{m v} $ Given, K.E of electron $=3.0 \times 10^{-25} \mathrm{~J}$ $ \begin{array}{l} \text { Since K.E.= } \frac{1}{2} m v^{2} \therefore...
Calculate the wavelength of an electron moving with a velocity of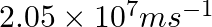
As per de Broglie’s equation, $ \lambda=\frac{h}{m v} $ Where, $\lambda$ denotes thr wavelength of the moving particle $\mathrm{m}$ is the mass of the particle $v$ denotes the velocity of the...
The electron energy in hydrogen atom is given by 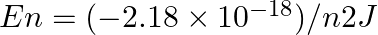 . Calculate the energy required to remove an electron completely from the n = 2 orbit. What is the longest wavelength of light in cm that can be used to cause this transition?
. Calculate the energy required to remove an electron completely from the n = 2 orbit. What is the longest wavelength of light in cm that can be used to cause this transition?
Required energy for the ionization from $\mathrm{n}=2$ is: $ \begin{array}{l} \Delta E=E_{\infty}-E_{2} \\ =\left[\left(\frac{-\left(2.18 \times...
What is the energy in joules, required to shift the electron of the hydrogen atom from the first Bohr orbit to the fifth Bohr orbit and what is the wavelength of the light emitted when the electron returns to the ground state? The ground state electron energy is 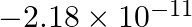 ergs. The ground-state electron energy is
ergs. The ground-state electron energy is 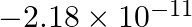 ergs.
ergs.
$ E_{5}=\frac{-\left(2.18 \times 10^{-18}\right) Z^{2}}{(n)^{2}} $ Where, $Z$ denotes the atom's atomic number Ground state energy $=-2.18 \times 10^{-11}$ ergs $=-2.18 \times 10^{-11} \times...
Calculate the wavenumber for the longest wavelength transition in the Balmer series of atomic hydrogen.
The Balmer series of the hydrogen emission spectrum, ni = 2. Hence, wavenumber expression ν is: $ \bar{\nu}=\left[\frac{1}{(2)^{2}}-\frac{1}{n_{f}^{2}}\right]\left(1.097 \times 10^{7}...
(i) The energy associated with the first orbit in the hydrogen atom is 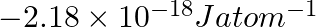 . What is the energy associated with the fifth orbit? (ii) Calculate the radius of Bohr’s fifth orbit for the hydrogen atom.
. What is the energy associated with the fifth orbit? (ii) Calculate the radius of Bohr’s fifth orbit for the hydrogen atom.
(i) Energy associated with the fifth orbit of hydrogen atom is calculated as: $E_{5}=\frac{-\left(2.18 \times 10^{-18}\right)}{(5)^{2}}=\frac{-\left(2.18 \times 10^{-18}\right)}{25}=-8.72 \times...
Glycogen is a branched-chain polymer of α-D-glucose units in which chain is formed by C1—C4 glycosidic linkage whereas branching occurs by the formation of C1-C6 glycosidic linkage. Structure of glycogen is similar to __________.
(i) Amylose
(ii) Amylopectin
(iii) Cellulose
(iv) Glucose
Option (ii) is the answer. Glycogen has a similar structure to amylopeptin. It's an a-D glucose unit branched chain polymer with C1-C4 glycosidic linkage for chain formation and C1-C6 glycosidic...
Which of the following polymer is stored in the liver of animals? (i) Amylose (ii) Cellulose (iii) Amylopectin (iv) Glycogen
Option (iv) is the answer. Glycogen is a type of sugar that is stored in the liver of mammals.
Sucrose (cane sugar) is a disaccharide. One molecule of sucrose on hydrolysis gives _________.
(i) 2 molecules of glucose
(ii) 2 molecules of glucose + 1 molecule of fructose
(iii) 1 molecule of glucose + 1 molecule of fructose
(iv) 2 molecules of fructose
Option (iii) is the answer. Cane sugar (sucrose) is a disaccharide. When sucrose is hydrolyzed, one molecule of glucose and one molecule of fructose are produced.
Which of the following pairs represents anomers?
Option (C) is the answer. Anomers are isomers that differ only in the conformation of the hydroxyl group at C—1 and are known as - and -fonns.
Proteins are found to have two different types of secondary structures viz. α-helix and β-pleated sheet structure. α-helix structure of the protein is stabilised by : (i) Peptide bonds (ii) van der Waals forces (iii) Hydrogen bonds (iv) Dipole-dipole interactions
Option ( iii) is the answer. Hydrogen bonding help to keep the -helix structure of proteins stable. By twisting into a right-handed helix and hydrogen bonding the -NH group of each amino acid...
In disaccharides, if the reducing groups of monosaccharides i.e. aldehydic or ketonic groups are bonded, these are non-reducing sugars. Which of the following disaccharide is a non-reducing sugar?
Option (B) is the answer. This structure represents sucrose, in which the C1—C2 glycosidic bond connects -D glucose and -D-fructose. This is a non-reducing sugar since the reducing groups of glucose...
Which of the following acids is a vitamin? (i) Aspartic acid (ii) Ascorbic acid (iii) Adipic acid (iv) Saccharic acid
Option (ii) is the answer. Vitamin C is ascorbic acid. Amino acid aspartic acid is a kind of amino acid. Dicarboxylic acids include adipic acid and saccharic acid.
Dinucleotide is obtained by joining two nucleotides together by phosphodiester linkage. Between which carbon atoms of pentose sugars of nucleotides are Are these linkages present? (i) 5′ and 3′ (ii) 1′ and 5′ (iii) 5′ and 5′ (iv) 3′ and 3′
Option (i) is the answer. Between the pentose sugars of nucleotides, there are 5′ and 3′ connections.
Nucleic acids are the polymers of ______________. (i) Nucleosides (ii) Nucleotides (iii) Bases (iv) Sugars
Option (ii) is the answer. Nucleic acids are nucleotide polymers connected together by phosphodiester linkage.
Which of the following statements is not true about glucose?
(i) It is an aldohexose.
(ii) On heating with HI, it forms n-hexane.
(iii) It is present in furanose form.
(iv) It does not give 2,4-DNP test.
Option (iii) is the answer. It's found in the pyranose structure.
Each polypeptide is a protein has amino acids linked with each other in a specific sequence. This sequence of amino acids is said to be ____________.
(i) primary structure of proteins.
(ii) secondary structure of proteins.
(iii) the tertiary structure of proteins.
(iv) quaternary structure of proteins.
Option (i) is the answer. The main structure of proteins is the sequence of amino acids in a polypeptide chain.
DNA and RNA contain four bases each. Which of the following bases is not present in RNA? (i) Adenine (ii) Uracil (iii) Thymine (iv) Cytosine
Option (iii) is the answer. Adenine, guanine, thymine, and cytosine are the four bases found in DNA. Adenine, uracil, guanine, and cytosine are the four bases found in RNA. As a result, thymine is...
Which of the following B group vitamins can be stored in our body? (i) Vitamin B1 (ii) Vitamin B2 (iii) Vitamin B6 (iv) Vitamin B12
Option (iv) is the answer. Because vitamin B12 is water insoluble, it can be stored in the body.
Which of the following bases is not present in DNA? (i) Adenine (ii) Thymine (iii) Cytosine (iv) Uracil
Option (iv) is the answer. In DNA, uracil is absent; instead, thymine is present.
Three cyclic structures of monosaccharides are given below which of these are anomers.
(i) I and II
(ii) II and III
(iii) I and III
(iv) III is anomer of I and II
Option (i) is the answer. Anomers are cyclic configurations of monosaccharides that differ in structure at carbon-1. I and II are anomers in this case because they differ solely in carbon-1.
Which of the following reactions of glucose can be explained only by its cyclic structure?
(i) Glucose forms pentaacetate.
(ii) Glucose reacts with hydroxylamine to form an oxime.
(iii) Pentaacetate of glucose does not react with hydroxylamine.
(iv) Glucose is oxidised by nitric acid to gluconic acid
Option (iii) is the answer. The absence of a free -CHO group is indicated by the fact that glucose pentaacetate does not react with hydroxylamine. Only the cyclic nature of glucose may explain this...
Optical rotations of some compounds along with their structures are given below which of them have D configuration.
(i) I, II, III
(ii) II, III
(iii) I, II
(iv) III
Option (i) is the answer. The -OH group is on the lowest asymmetric carbon on the right side of the I, II, and III structures, which is similar to (+) glyceraldehyde.
Structure of a disaccharide formed by glucose and fructose is given below. Identify anomeric carbon atoms in monosaccharide units.
(i) ‘a’ carbon of glucose and ‘a’ carbon of fructose.
(ii) ‘a’ carbon of glucose and ‘e’ carbon of fructose.
(iii) ‘a’ carbon of glucose and ‘b’ carbon of fructose.
(iv) ‘f’ carbon of glucose and ‘f ’ carbon of fructose.
Option (iii) is the answer. Anomeric carbon is carbon that is next to an oxygen atom in the cyclic structure of glucose or fructose. 'a' and 'b' are next to the oxygen atom, as illustrated in the...
Three structures are given below in which two glucose units are linked. Which of these linkages between glucose, units are between C1 and C4 and which linkages are between C1 and C6?
(i) (A) is between C1 and C4, (B) and (C) is between C1 and C6
(ii) (A) and (B) are between C1 and C4, (C) is between C1 and C6
(iii) (A) and (C) is between C1 and C4, (B) is between C1 and C6
(iv) (A) and (C) is between C1 and C6, (B) is between C1 and C4
Option (iii) is the answer (A) and (C) are in the Cl-C4 range, while (B) is in the Cl-C6 range.
Carbohydrates are classified on the basis of their behaviour on hydrolysis and also as reducing or non-reducing sugar. Sucrose is a __________. (i) monosaccharide (ii) disaccharide (iii) reducing sugar (iv) non-reducing sugar
Option (ii) and (iv) are the answers. Sucrose is a non-reducing sugar and a disaccharide.
Proteins can be classified into two types on the basis of their molecular shape i.e., fibrous proteins and globular proteins. Examples of globular proteins are : (i) Insulin (ii) Keratin (iii) Albumin (iv) Myosin
Option (i) and (iii) are the answers Globulular protein is the structure that develops when a chain of polypeptides coils around to form a spherical shape. Insulin and albumin, for example, are...
Which of the following carbohydrates are branched polymer of glucose?
(i) Amylose
(ii) Amylopectin
(iii) Cellulose
(iv) Glycogen
Option (i) and (iv) are the answers. Amylopectin and glycogen are both glucose branching polymers.
Amino acids are classified as acidic, basic or neutral depending upon the relative number of amino and carboxyl groups in their molecule. Which of the following is acidic?
Option (ii) and (iv) are the answers. Acidic amino acids have more than one -COOH group one against the –NH2 group.
Lysine, is _______________.
(i) α-Amino acid
(ii) Basic amino acid
(iii) Amino acid synthesised in the body
(iv) β-Amino acid
Option (i), (ii) and (iii) are the answers. (a)Lysine is a kind of amino acid with the structural formula . (b) Because the number of NH2 groups (2) is more than the number of COOH groups, it is a...
Which of the following monosaccharides are present as five-membered cyclic structure (furanose structure)? (i) Ribose (ii) Glucose (iii) Fructose (iv) Galactose
Option (i) and (iii) are the answers. The five-membered cyclic structure of ribose and fructose is shown (furanose structures). They have a five-membered ring, similar to the foran compound....
In fibrous proteins, polypeptide chains are held together by ___________.
(i) van der Waals forces
(ii) disulphide linkage
(iii) electrostatic forces of attraction
(iv) hydrogen bonds
Option (ii) and (iv) are the answers. Disulphide linkage and hydrogen bonding hold polypeptide chains together in fibrous proteins.
Which of the following are purine bases?
(i) Guanine
(ii) Adenine
(iii) Thymine
(iv) Uracil
Option (i) and (ii) are the answers. Purines are made up of a six-membered nitrogen-containing ring fused together with a five-membered nitrogen-containing ring. Purine bases guanine and adenine...
Which of the following terms are correct about enzyme?
(i) Proteins
(ii) Dinucleotides
(iii) Nucleic acids
(iv) Biocatalysts
Option (i) and (iv) are the answers. Enzymes are protein molecules that act as biocatalysts in the body's chemical reactions.
Name the sugar present in milk. How many monosaccharide units are present in it? What are such oligosaccharides called?
Lactose is the sugar found in milk. Glucose and galactose are two monosaccharides found in lactose. Disaccharides are oligosaccharides that include two monosaccharide units.
How do you explain the presence of all the six carbon atoms in glucose in a straight chain?
When glucose is heated with HI for a long time, n-hexane develops, implying that all six carbon atoms are connected in a straight chain.
In nucleoside, a base is attached at 1C position of the sugar moiety. A nucleotide is formed by linking the phosphoric acid unit to the sugar unit of a nucleoside. At which position of sugar unit is the phosphoric acid linked in a nucleoside to give a nucleotide?
When a nitrogenous base is connected to the 1' position of a five-carbon sugar, a nucleoside is produced. The 5' carbon of the sugar in a nucleoside molecule is bonded to the 5' carbon of the sugar...
Name the linkage connecting monosaccharide units in polysaccharides.
Glycosidic linkages connect the monosaccharide units of polysaccharides. When an oxide bond is created between two monosaccharide units with the loss of a water molecule, it is called a glycosidic...
Under what conditions glucose is converted to gluconic and saccharic acid?
When glucose is treated with a mild oxidising agent like Br2 water, it is transformed to gluconic acid, a six-carbon carboxylic acid. When glucose is treated with nitric acid, it is transformed to...
Monosaccharides contain carbonyl group hence are classified, as aldose or ketose. The number of carbon atoms present in the monosaccharide molecule is also considered for classification. In which class of monosaccharide will you place fructose?
Carbonyl groups can be found in monosaccharides. As a result, they're categorised as either aldose or ketose. Aldose refers to monosaccharides that contain an aldehyde group. Ketose refers to...
The letters ‘D’ or ‘L’ before the name of a stereoisomer of a compound indicates the correlation of configuration of that particular stereoisomer. This refers to their relationship with one of the isomers of glyceraldehyde. Predict whether the following compound has ‘D’ or ‘L’ configuration.
On the left side of the C5 carbon atom, the –OH group is connected. As a result, the provided compound is in the 'L' configuration.
Aldopentoses named as ribose and 2-deoxyribose are found in nucleic acids. What is their relative configuration?
D-configuration is the configuration of both aldopentoses. -D-ribose is ribose, while -D-2-deoxyribose is 2-deoxyribose.
Which sugar is called invert sugar? Why is it called so?
Invert sugar is another name for sucrose. It comes from sugarcane and sugarbeet and is a naturally occurring sugar. When sucrose is hydrolyzed, the sign of rotation changes from Dextro (+) to laevo...
Amino acids can be classified as α-, β-, -, δ- and so on depending upon the relative position of the amino group concerning the carboxyl group. Which type of amino acids forms polypeptide chain in proteins?
The sort of amino acids that make up a polypeptide chain are -amino acids and alpha-amino acids, where the amino acid is linked to the -carbon in the molecule.
α-Helix is a secondary structure of proteins formed by twisting of the polypeptide chain into right-handed screw-like structures. Which type of interactions is responsible for making the a-helix structure stable?
The –NH group of each amino acid residue hydrogen is bound to the –C=O of an adjacent turn of the helix, forming a right-handed screw helix shape.
Some enzymes are named after the reaction, where they are used. What name is given to the class of enzymes which catalyse the oxidation of one substrate with simultaneous reduction of another substrate?
Enzyme oxidoreductases is the name given to a group of enzymes that catalyse redox processes. Alcohol Dehydrogenase, for example, aids in the reduction of alcohol levels in the human body when...
During curdling of milk, what happens to sugar present in it?
The sugar found in milk, lactose, is transformed to lactic acid during curdling, which is produced by bacteria.
How do you explain the presence of five —OH groups in the glucose molecule?
When glucose is acetylated using acetic anhydride (CH3CO)2O in the presence of ZnCl2, glucose pentaacetate is formed, confirming the presence of five –OH groups.
